Selective FPR2 Agonism Promotes a Proresolution Macrophage Phenotype and Improves Cardiac Structure-Function Post Myocardial Infarction
- PMID: 34466754
- PMCID: PMC8385569
- DOI: 10.1016/j.jacbts.2021.07.007
Selective FPR2 Agonism Promotes a Proresolution Macrophage Phenotype and Improves Cardiac Structure-Function Post Myocardial Infarction
Abstract
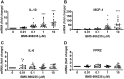
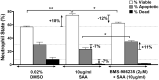
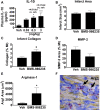
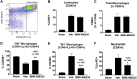
Dysregulated inflammation following myocardial infarction (MI) leads to maladaptive healing and remodeling. The study characterized and evaluated a selective formyl peptide receptor 2 (FPR2) agonist BMS-986235 in cellular assays and in rodents undergoing MI. BMS-986235 activated G proteins and promoted β-arrestin recruitment, enhanced phagocytosis and neutrophil apoptosis, regulated chemotaxis, and stimulated interleukin-10 and monocyte chemoattractant protein-1 gene expression. Treatment with BMS-986235 improved mouse survival, reduced left ventricular area, reduced scar area, and preserved wall thickness. Treatment increased macrophage arginase-1 messenger RNA and CD206 receptor levels indicating a proresolution phenotype. In rats following MI, BMS-986235 preserved viable myocardium, attenuated left ventricular remodeling, and increased ejection fraction relative to control animals. Therefore, FPR2 agonism improves post-MI healing, limits remodeling and preserves function, and may offer an innovative therapeutic option to improve outcomes.
Keywords: BRET, bioluminescence resonance energy transfer; EC50, half maximal effective concentration; FPR2; FPR2, formyl peptide receptor 2; HF; HF, heart failure; I/R, ischemia-reperfusion; IL, interleukin; KO, knockout; LPS, lipopolysaccharide; LV, left ventricle/ventricular; MCP, monocyte chemoattractant protein; MI; MI, myocardial infarction; SAA, serum amyloid A; TNF, tumor necrosis factor; WT, wild-type; formyl peptide receptor 2; heart failure; mRNA, messenger RNA; myocardial infarction; resolution.
© 2021 The Authors.
Conflict of interest statement
This work was supported by Bristol Myers Squibb (Princeton, New Jersey, USA). All authors are employees of Bristol Myers Squibb or affiliates via collaboration or contract research.
Figures




Similar articles
-
Molecular Mechanisms of Desensitization Underlying the Differential Effects of Formyl Peptide Receptor 2 Agonists on Cardiac Structure-Function Post Myocardial Infarction.ACS Pharmacol Transl Sci. 2022 Sep 14;5(10):892-906. doi: 10.1021/acsptsci.2c00042. eCollection 2022 Oct 14. ACS Pharmacol Transl Sci. 2022. PMID: 36268126 Free PMC article.
-
Preservation of Post-Infarction Cardiac Structure and Function via Long-Term Oral Formyl Peptide Receptor Agonist Treatment.JACC Basic Transl Sci. 2019 Nov 13;4(8):905-920. doi: 10.1016/j.jacbts.2019.07.005. eCollection 2019 Dec. JACC Basic Transl Sci. 2019. PMID: 31909300 Free PMC article.
-
Inhibition of FPR2 impaired leukocytes recruitment and elicited non-resolving inflammation in acute heart failure.Pharmacol Res. 2019 Aug;146:104295. doi: 10.1016/j.phrs.2019.104295. Epub 2019 Jun 16. Pharmacol Res. 2019. PMID: 31216426 Free PMC article.
-
Cardiac overexpression of monocyte chemoattractant protein-1 in transgenic mice prevents cardiac dysfunction and remodeling after myocardial infarction.Circ Res. 2006 Oct 13;99(8):891-9. doi: 10.1161/01.RES.0000246113.82111.2d. Epub 2006 Sep 21. Circ Res. 2006. PMID: 16990567
-
Formyl peptide receptor 2 and heart disease.Semin Immunol. 2022 Jan;59:101602. doi: 10.1016/j.smim.2022.101602. Epub 2022 Mar 8. Semin Immunol. 2022. PMID: 35277300 Review.
Cited by
-
Molecular Mechanisms of Desensitization Underlying the Differential Effects of Formyl Peptide Receptor 2 Agonists on Cardiac Structure-Function Post Myocardial Infarction.ACS Pharmacol Transl Sci. 2022 Sep 14;5(10):892-906. doi: 10.1021/acsptsci.2c00042. eCollection 2022 Oct 14. ACS Pharmacol Transl Sci. 2022. PMID: 36268126 Free PMC article.
-
Leveraging FPR2 Agonists to Resolve Inflammation and Improve Outcomes Following Myocardial Infarction.JACC Basic Transl Sci. 2021 Aug 23;6(8):690-692. doi: 10.1016/j.jacbts.2021.07.009. eCollection 2021 Aug. JACC Basic Transl Sci. 2021. PMID: 34466755 Free PMC article.
-
Single-Cell Analysis Reveals the Role of the Neuropeptide Receptor FPR2 in Monocytes in Kawasaki Disease: A Bioinformatic Study.Dis Markers. 2022 Jun 1;2022:1666240. doi: 10.1155/2022/1666240. eCollection 2022. Dis Markers. 2022. PMID: 35692878 Free PMC article.
-
Fpr2-/- Mice Developed Exacerbated Alcohol-Associated Liver Disease.Biology (Basel). 2023 Apr 23;12(5):639. doi: 10.3390/biology12050639. Biology (Basel). 2023. PMID: 37237453 Free PMC article.
-
Loss of Histone Methyltransferase KMT2D Attenuates Angiogenesis in the Ischemic Heart by Inhibiting the Transcriptional Activation of VEGF-A.J Cardiovasc Transl Res. 2023 Oct;16(5):1032-1049. doi: 10.1007/s12265-023-10373-x. Epub 2023 Mar 22. J Cardiovasc Transl Res. 2023. PMID: 36947365 Free PMC article.
References
-
- Adamo L., Rocha-Resende C., Prabhu S.D., Mann D.L. Reappraising the role of inflammation in heart failure. Nat Rev Cardiol. 2020;17:269–285. - PubMed
-
- Hammerman H., Kloner R.A., Hale S., Schoen F.J., Braunwald E. Dose-dependent effects of short-term methylprednisolone on myocardial infarct extent, scar formation, and ventricular function. Circulation. 1983;68:446–452. - PubMed
-
- Roberts R., DeMello V., Sobel B.E. Deleterious effects of methylprednisolone in patients with myocardial infarction. Circulation. 1976;53:I204–I206. - PubMed
-
- Saito T., Rodger I.W., Hu F., Robinson R., Huynh T., Giaid A. Inhibition of COX pathway in experimental myocardial infarction. J Mol Cell Cardiol. 2004;37:71–77. - PubMed
-
- Mann D.L., McMurray J.J.V., Packer M. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL) Circulation. 2004;109:1594–1602. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

