The inhibitory effects of Orengedokuto on inducible PGE2 production in BV-2 microglial cells
- PMID: 34458607
- PMCID: PMC8377439
- DOI: 10.1016/j.heliyon.2021.e07759
The inhibitory effects of Orengedokuto on inducible PGE2 production in BV-2 microglial cells
Abstract
Background and aim: Reactive microglia has been associated with neuroinflammation caused by the production of proinflammatory molecules such as cytokines, nitric oxide, and prostaglandins. The overexpression of these molecules may provoke neuronal damage that can cause neurodegenerative diseases. A traditional herbal medicine, Orengedokuto (OGT), has been widely used for treating inflammation-related diseases. However, how it influences neuroinflammation remains poorly understood.
Experimental procedure: This study investigated the effects of OGT on inflammatory molecule induction in BV-2 microglial cells using real-time RT-PCR and ELISA. An in vivo confirmation of these effects was then performed in mice.
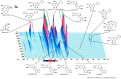
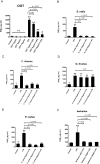
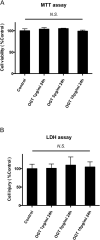
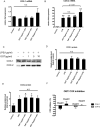
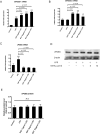
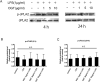
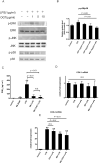
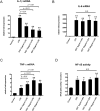
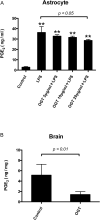
Results and conclusion: OGT showed dose-dependent inhibition of prostaglandin E2 (PGE2) production in BV-2 cells stimulated with lipopolysaccharide (LPS). To elucidate the mechanism of PGE2 inhibition, we examined cyclooxygenases (COXs) and found that OGT did not suppress COX-1 expression or inhibit LPS-induced COX-2 upregulation at either the transcriptional or translational levels. In addition, OGT did not inhibit COX enzyme activities within the concentration that inhibited PGE2 production, suggesting that the effect of OGT is COX-independent. The inhibitory effects of OGT on PGE2 production in BV-2 cells were experimentally replicated in primary cultured astrocytes and mice brains. OGT can be useful in the treatment of neuroinflammatory diseases by modulating PGE2 expression.
Keywords: Microglia; Neuroinflammation; Orengedokuto; PGE2; p38 MAPK.
© 2021 The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Novel celecoxib analogues inhibit glial production of prostaglandin E2, nitric oxide, and oxygen radicals reverting the neuroinflammatory responses induced by misfolded prion protein fragment 90-231 or lipopolysaccharide.Pharmacol Res. 2016 Nov;113(Pt A):500-514. doi: 10.1016/j.phrs.2016.09.010. Epub 2016 Sep 22. Pharmacol Res. 2016. PMID: 27667770
-
Inhibitory effect of 4-O-methylhonokiol on lipopolysaccharide-induced neuroinflammation, amyloidogenesis and memory impairment via inhibition of nuclear factor-kappaB in vitro and in vivo models.J Neuroinflammation. 2012 Feb 19;9:35. doi: 10.1186/1742-2094-9-35. J Neuroinflammation. 2012. PMID: 22339795 Free PMC article.
-
Cytosolic phospholipase A2 plays a crucial role in ROS/NO signaling during microglial activation through the lipoxygenase pathway.J Neuroinflammation. 2015 Oct 31;12:199. doi: 10.1186/s12974-015-0419-0. J Neuroinflammation. 2015. PMID: 26520095 Free PMC article.
-
Regulation of prostanoid synthesis in microglial cells and effects of prostaglandin E2 on microglial functions.Biochimie. 1998 Nov;80(11):899-904. doi: 10.1016/s0300-9084(00)88886-0. Biochimie. 1998. PMID: 9893949 Review.
-
Is cyclooxygenase-1 involved in neuroinflammation?J Neurosci Res. 2021 Nov;99(11):2976-2998. doi: 10.1002/jnr.24934. Epub 2021 Aug 4. J Neurosci Res. 2021. PMID: 34346520 Free PMC article. Review.
Cited by
-
From tryptamine to the discovery of efficient multi-target directed ligands against cholinesterase-associated neurodegenerative disorders.Front Pharmacol. 2022 Nov 28;13:1036030. doi: 10.3389/fphar.2022.1036030. eCollection 2022. Front Pharmacol. 2022. PMID: 36518670 Free PMC article.
-
Exploring the role of COX-2 in Alzheimer's disease: Potential therapeutic implications of COX-2 inhibitors.Saudi Pharm J. 2023 Sep;31(9):101729. doi: 10.1016/j.jsps.2023.101729. Epub 2023 Aug 7. Saudi Pharm J. 2023. PMID: 37638222 Free PMC article. Review.
References
-
- Gehrmann J., Matsumoto Y., Kreutzberg G.W. Microglia: intrinsic immuneffector cell of the brain. Brain Res. Rev. 1995;20:269–287. - PubMed
-
- Christensen R.N., Ha B.K., Sun F., Bresnahan J.C., Beattie M.S. Kainate induces rapid redistribution of the actin cytoskeleton in ameboid microglia. J. Neurosci. Res. 2006;84:170–181. - PubMed
-
- Aloisi F. Immune function of microglia. Glia. 2001;36:165–179. - PubMed
-
- Widmann C.N., Heneka M.T. Long-term cerebral consequences of sepsis. Lancet Neurol. 2014;13:630–636. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

