Efficacy and Safety of a Modified Vaccinia Ankara-NP+M1 Vaccine Combined with QIV in People Aged 65 and Older: A Randomised Controlled Clinical Trial (INVICTUS)
- PMID: 34451976
- PMCID: PMC8402379
- DOI: 10.3390/vaccines9080851
Efficacy and Safety of a Modified Vaccinia Ankara-NP+M1 Vaccine Combined with QIV in People Aged 65 and Older: A Randomised Controlled Clinical Trial (INVICTUS)
Abstract
Background: Pre-existing T cell responses to influenza have been correlated with improved clinical outcomes in natural history and human challenge studies. We aimed to determine the efficacy, safety and immunogenicity of a T-cell directed vaccine in older people.
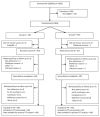
Methods: This was a multicentre, participant- and safety assessor-blinded, randomised, placebo-controlled trial of the co-administration of Modified Vaccinia Ankara encoding nucleoprotein and matrix protein 1 (MVA-NP+M1) and annual influenza vaccine in participants ≥ 65. The primary outcome was the number of days with moderate or severe influenza-like symptoms (ILS) during the influenza season.
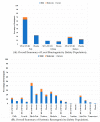
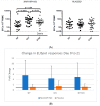
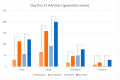
Results: 846 of a planned 2030 participants were recruited in the UK prior to, and throughout, the 2017/18 flu season. There was no evidence of a difference in the reported rates of days of moderate or severe ILS during influenza-like illness episodes (unadjusted OR = 0.95, 95% CI: 0.54-1.69; adjusted OR = 0.91, 95% CI: 0.51-1.65). The trial was stopped after one season due to a change in the recommended annual flu vaccine, for which safety of the new combination had not been established. More participants in the MVA-NP+M1 group had transient moderate or severe pain, redness, and systemic responses in the first seven days.
Conclusion: The MVA-NP+M1 vaccine is well tolerated in those aged 65 years and over. Larger trials would be needed to determine potential efficacy.
Keywords: MVA; elderly; influenza; nucleoprotein; vaccination.
Conflict of interest statement
T.G.E., E.E.-V., L.B. and C.E. were employed by Vaccitech Ltd., the sponsor of the study. S.G. owns stock in Vaccitech Ltd. K.R. received consultancy income from Vaccitech Ltd.
Figures
Similar articles
-
Efficacy and safety of a universal influenza A vaccine (MVA-NP+M1) in adults when given after seasonal quadrivalent influenza vaccine immunisation (FLU009): a phase 2b, randomised, double-blind trial.Lancet Infect Dis. 2022 Jun;22(6):857-866. doi: 10.1016/S1473-3099(21)00702-7. Epub 2022 Mar 16. Lancet Infect Dis. 2022. PMID: 35305317 Clinical Trial.
-
A phase IIb study to determine the safety and efficacy of candidate INfluenza Vaccine MVA-NP+M1 in combination with licensed Ina CTivated infl Uenza vaccine in adult S aged 65 years and above (INVICTUS): a study protocol.F1000Res. 2019 May 23;8:719. doi: 10.12688/f1000research.19090.1. eCollection 2019. F1000Res. 2019. PMID: 32089822 Free PMC article. Clinical Trial.
-
A T cell-inducing influenza vaccine for the elderly: safety and immunogenicity of MVA-NP+M1 in adults aged over 50 years.PLoS One. 2012;7(10):e48322. doi: 10.1371/journal.pone.0048322. Epub 2012 Oct 31. PLoS One. 2012. PMID: 23118984 Free PMC article. Clinical Trial.
-
Influenza virus vaccine live intranasal--MedImmune vaccines: CAIV-T, influenza vaccine live intranasal.Drugs R D. 2003;4(5):312-9. doi: 10.2165/00126839-200304050-00007. Drugs R D. 2003. PMID: 12952502 Review.
-
Immunogenicity and safety of inactivated quadrivalent influenza vaccine in adults: A systematic review and meta-analysis of randomised controlled trials.Vaccine. 2016 Jul 29;34(35):4092-4102. doi: 10.1016/j.vaccine.2016.06.064. Epub 2016 Jul 2. Vaccine. 2016. PMID: 27381642 Review.
Cited by
-
The Role of Nucleoprotein in Immunity to Human Negative-Stranded RNA Viruses-Not Just Another Brick in the Viral Nucleocapsid.Viruses. 2022 Mar 3;14(3):521. doi: 10.3390/v14030521. Viruses. 2022. PMID: 35336928 Free PMC article. Review.
-
Nucleoprotein as a Promising Antigen for Broadly Protective Influenza Vaccines.Vaccines (Basel). 2023 Nov 23;11(12):1747. doi: 10.3390/vaccines11121747. Vaccines (Basel). 2023. PMID: 38140152 Free PMC article. Review.
-
Nanovaccine Delivery Approaches and Advanced Delivery Systems for the Prevention of Viral Infections: From Development to Clinical Application.Pharmaceutics. 2021 Dec 5;13(12):2091. doi: 10.3390/pharmaceutics13122091. Pharmaceutics. 2021. PMID: 34959372 Free PMC article. Review.
-
Vaccines for Respiratory Viruses-COVID and Beyond.Vaccines (Basel). 2024 Aug 22;12(8):936. doi: 10.3390/vaccines12080936. Vaccines (Basel). 2024. PMID: 39204059 Free PMC article. Review.
References
-
- [(accessed on 7 July 2021)]; Available online: https://www.who.int/influenza/Global_Influenza_Strategy_2019_2030_Summar....
-
- Dawood F.S., Iuliano A.D., Reed C., Meltzer M.I., Shay D.K., Cheng P.Y., Bandaranayake D., Breiman R.F., Brooks W.A., Buchy P., et al. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: A modelling study. Lancet Infect. Dis. 2012;12:687–695. doi: 10.1016/S1473-3099(12)70121-4. - DOI - PubMed
-
- Hayward A.C., Wang L., Goonetilleke N., Fragaszy E.B., Bermingham A., Copas A., Dukes O., Millett E.R., Nazareth I., Nguyen-Van-Tam J.S., et al. Natural T Cell-mediated Protection against Seasonal and Pandemic Influenza. Results of the Flu Watch Cohort Study. Am. J. Respir. Crit. Care Med. 2015;191:1422–1431. doi: 10.1164/rccm.201411-1988OC. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

