The Glyoxalase System in Age-Related Diseases: Nutritional Intervention as Anti-Ageing Strategy
- PMID: 34440621
- PMCID: PMC8393707
- DOI: 10.3390/cells10081852
The Glyoxalase System in Age-Related Diseases: Nutritional Intervention as Anti-Ageing Strategy
Abstract
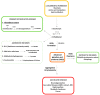
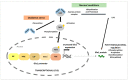
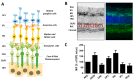
The glyoxalase system is critical for the detoxification of advanced glycation end-products (AGEs). AGEs are toxic compounds resulting from the non-enzymatic modification of biomolecules by sugars or their metabolites through a process called glycation. AGEs have adverse effects on many tissues, playing a pathogenic role in the progression of molecular and cellular aging. Due to the age-related decline in different anti-AGE mechanisms, including detoxifying mechanisms and proteolytic capacities, glycated biomolecules are accumulated during normal aging in our body in a tissue-dependent manner. Viewed in this way, anti-AGE detoxifying systems are proposed as therapeutic targets to fight pathological dysfunction associated with AGE accumulation and cytotoxicity. Here, we summarize the current state of knowledge related to the protective mechanisms against glycative stress, with a special emphasis on the glyoxalase system as the primary mechanism for detoxifying the reactive intermediates of glycation. This review focuses on glyoxalase 1 (GLO1), the first enzyme of the glyoxalase system, and the rate-limiting enzyme of this catalytic process. Although GLO1 is ubiquitously expressed, protein levels and activities are regulated in a tissue-dependent manner. We provide a comparative analysis of GLO1 protein in different tissues. Our findings indicate a role for the glyoxalase system in homeostasis in the eye retina, a highly oxygenated tissue with rapid protein turnover. We also describe modulation of the glyoxalase system as a therapeutic target to delay the development of age-related diseases and summarize the literature that describes the current knowledge about nutritional compounds with properties to modulate the glyoxalase system.
Keywords: aging; glycative stress; glyoxalase system; proteotoxicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The Role of Glyoxalase in Glycation and Carbonyl Stress Induced Metabolic Disorders.Curr Protein Pept Sci. 2020;21(9):846-859. doi: 10.2174/1389203721666200505101734. Curr Protein Pept Sci. 2020. PMID: 32368974 Review.
-
RAGE and glyoxalase in kidney disease.Glycoconj J. 2016 Aug;33(4):619-26. doi: 10.1007/s10719-016-9689-8. Epub 2016 Jun 6. Glycoconj J. 2016. PMID: 27270765 Review.
-
Glyoxalase system: A systematic review of its biological activity, related-diseases, screening methods and small molecule regulators.Biomed Pharmacother. 2020 Nov;131:110663. doi: 10.1016/j.biopha.2020.110663. Epub 2020 Aug 25. Biomed Pharmacother. 2020. PMID: 32858501
-
Glyoxalase I retards renal senescence.Am J Pathol. 2011 Dec;179(6):2810-21. doi: 10.1016/j.ajpath.2011.08.023. Epub 2011 Oct 12. Am J Pathol. 2011. PMID: 22001178 Free PMC article.
-
Advanced glycation end products in the pathogenesis of chronic kidney disease.Kidney Int. 2018 Apr;93(4):803-813. doi: 10.1016/j.kint.2017.11.034. Epub 2018 Feb 22. Kidney Int. 2018. PMID: 29477239 Review.
Cited by
-
Methylglyoxal and Its Adducts: Induction, Repair, and Association with Disease.Chem Res Toxicol. 2022 Oct 17;35(10):1720-1746. doi: 10.1021/acs.chemrestox.2c00160. Epub 2022 Oct 5. Chem Res Toxicol. 2022. PMID: 36197742 Free PMC article. Review.
-
Glyoxalase I Assay as a Possible Tool for Evaluation of Biological Activity of Antioxidant-Rich Plant Extracts.Plants (Basel). 2023 Mar 3;12(5):1150. doi: 10.3390/plants12051150. Plants (Basel). 2023. PMID: 36904010 Free PMC article.
-
Molecular Assessment of Methylglyoxal-Induced Toxicity and Therapeutic Approaches in Various Diseases: Exploring the Interplay with the Glyoxalase System.Life (Basel). 2024 Feb 17;14(2):263. doi: 10.3390/life14020263. Life (Basel). 2024. PMID: 38398772 Free PMC article. Review.
-
Advanced Glycation Endproducts: A Marker of Long-term Exposure to Glycemia.J Diabetes Sci Technol. 2024 Mar 25:19322968241240436. doi: 10.1177/19322968241240436. Online ahead of print. J Diabetes Sci Technol. 2024. PMID: 38525944 Free PMC article.
-
Autophagy and Glycative Stress: A Bittersweet Relationship in Neurodegeneration.Front Cell Dev Biol. 2021 Dec 23;9:790479. doi: 10.3389/fcell.2021.790479. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 35004686 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

