Celastrol exerts a neuroprotective effect by directly binding to HMGB1 protein in cerebral ischemia-reperfusion
- PMID: 34372857
- PMCID: PMC8353826
- DOI: 10.1186/s12974-021-02216-w
Celastrol exerts a neuroprotective effect by directly binding to HMGB1 protein in cerebral ischemia-reperfusion
Abstract
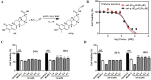
Background: Celastrol (cel) was one of the earliest isolated and identified chemical constituents of Tripterygium wilfordii Hook. f. Based on a cel probe (cel-p) that maintained the bioactivity of the parent compound, the targets of cel in cerebral ischemia-reperfusion (I/R) injury were comprehensively analyzed by a quantitative chemical proteomics method.
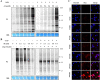
Methods: We constructed an oxygen-glucose deprivation (OGD) model in primary rat cortical neurons and a middle cerebral artery occlusion (MCAO) model in adult rats to detect the direct binding targets of cel in cerebral I/R. By combining various experimental methods, including tandem mass tag (TMT) labeling, mass spectrometry, and cellular thermal shift assay (CETSA), we revealed the targets to which cel directly bound to exert neuroprotective effects.
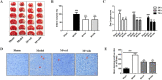
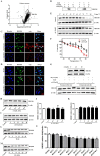
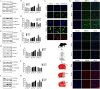
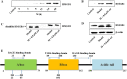
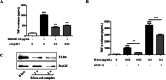
Results: We found that cel inhibited the proinflammatory activity of high mobility group protein 1 (HMGB1) by directly binding to it and then blocking the binding of HMGB1 to its inflammatory receptors in the microenvironment of ischemia and hypoxia. In addition, cel rescued neurons from OGD injury in vitro and decreased cerebral infarction in vivo by targeting HSP70 and NF-κB p65.
Conclusion: Cel exhibited neuroprotective and anti-inflammatory effects by targeting HSP70 and NF-κB p65 and directly binding to HMGB1 in cerebral I/R injury.
Keywords: Celastrol; Cerebral ischemia–reperfusion; Chemical proteomics; High mobility group protein 1; Target identification.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures


Similar articles
-
Withaferin A and Celastrol Overwhelm Proteostasis.Int J Mol Sci. 2023 Dec 27;25(1):367. doi: 10.3390/ijms25010367. Int J Mol Sci. 2023. PMID: 38203539 Free PMC article. Review.
-
Salvianolic Acid D Alleviates Cerebral Ischemia-Reperfusion Injury by Suppressing the Cytoplasmic Translocation and Release of HMGB1-Triggered NF-κB Activation to Inhibit Inflammatory Response.Mediators Inflamm. 2020 Jan 22;2020:9049614. doi: 10.1155/2020/9049614. eCollection 2020. Mediators Inflamm. 2020. PMID: 32410871 Free PMC article.
-
HMGB1-triggered inflammation inhibition of notoginseng leaf triterpenes against cerebral ischemia and reperfusion injury via MAPK and NF-κB signaling pathways.Biomolecules. 2019 Sep 20;9(10):512. doi: 10.3390/biom9100512. Biomolecules. 2019. PMID: 31547018 Free PMC article.
-
Lipidomic Profiling of Ipsilateral Brain and Plasma after Celastrol Post-Treatment in Transient Middle Cerebral Artery Occlusion Mice Model.Molecules. 2021 Jul 7;26(14):4124. doi: 10.3390/molecules26144124. Molecules. 2021. PMID: 34299399 Free PMC article.
-
Neuroprotective Effects of Celastrol in Neurodegenerative Diseases-Unscramble Its Major Mechanisms of Action and Targets.Aging Dis. 2022 Jun 1;13(3):815-836. doi: 10.14336/AD.2021.1115. eCollection 2022 Jun. Aging Dis. 2022. PMID: 35656110 Free PMC article. Review.
Cited by
-
Chemoproteomics-based profiling reveals potential antimalarial mechanism of Celastrol by disrupting spermidine and protein synthesis.Cell Commun Signal. 2024 Feb 20;22(1):139. doi: 10.1186/s12964-023-01409-5. Cell Commun Signal. 2024. PMID: 38378659 Free PMC article.
-
Withaferin A and Celastrol Overwhelm Proteostasis.Int J Mol Sci. 2023 Dec 27;25(1):367. doi: 10.3390/ijms25010367. Int J Mol Sci. 2023. PMID: 38203539 Free PMC article. Review.
-
Effect of Celastrol on LncRNAs and mRNAs Profiles of Cerebral Ischemia-Reperfusion Injury in Transient Middle Cerebral Artery Occlusion Mice Model.Front Neurosci. 2022 May 23;16:889292. doi: 10.3389/fnins.2022.889292. eCollection 2022. Front Neurosci. 2022. PMID: 35677353 Free PMC article.
-
Celastrol ameliorates hypoxic-ischemic brain injury in neonatal rats by reducing oxidative stress and inflammation.Pediatr Res. 2024 Dec;96(7):1681-1692. doi: 10.1038/s41390-024-03246-9. Epub 2024 May 20. Pediatr Res. 2024. PMID: 38763946 Free PMC article.
-
Celastrol targeting Nedd4 reduces Nrf2-mediated oxidative stress in astrocytes after ischemic stroke.J Pharm Anal. 2023 Feb;13(2):156-169. doi: 10.1016/j.jpha.2022.12.002. Epub 2023 Jan 7. J Pharm Anal. 2023. PMID: 36908855 Free PMC article.
References
-
- Zhang Y, Mao X, Li W, Chen W, Wang X, Ma Z, Lin N. Tripterygium wilfordii: an inspiring resource for rheumatoid arthritis treatment. Med Res Rev. 2020;41:1337–74. - PubMed
-
- Yizhi, Chen, Zhixiang, Gong, Xiangmei, Chen, Li, Tang, Xuezhi, Zhao. Tripterygium wilfordii Hook F (a traditional Chinese medicine) for primary nephrotic syndrome. Cochrane Database Syst Rev. 2013;(8). Art. No.:CD008568. - PubMed
-
- Lu Y, Liu Y, Zhou J, Li D, Gao W. Biosynthesis, total synthesis, structural modifications, bioactivity, and mechanism of action of the quinone-methide triterpenoid celastrol. Med Res Rev. 2021;41:1022–60. - PubMed
-
- Xu S, Feng Y, He W, Xu W, Xu W, Yang H, Li X. Celastrol in metabolic diseases: progress and application prospects. Pharmacol Res. 2021;167:105572. - PubMed
MeSH terms
Substances
Grants and funding
- ZXKT18003/the fundamental research funds for the central public welfare research institutes
- 2017ZX09101002-001-001-05/the Major National Science and Technology Program of China for Innovative Drug
- 2020YFA0908000, 2020YFA0908004, 2020YFA0908001/the national key research and development program of china
- 81903588, 81803456, 81702580, 82074098, 81841001 and 81803389/national natural science foundation of china
- 81903866/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Research Materials

