The Dual Role of a Polyvalent IgM/IgA-Enriched Immunoglobulin Preparation in Activating and Inhibiting the Complement System
- PMID: 34356880
- PMCID: PMC8301464
- DOI: 10.3390/biomedicines9070817
The Dual Role of a Polyvalent IgM/IgA-Enriched Immunoglobulin Preparation in Activating and Inhibiting the Complement System
Abstract
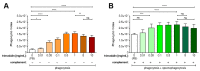
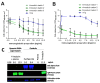
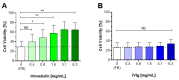
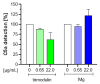
Activation of the complement system is important for efficient clearance of a wide variety of pathogens via opsonophagocytosis, or by direct lysis via complement-dependent cytotoxicity (CDC). However, in severe infections dysregulation of the complement system contributes to hyperinflammation. The influence of the novel IgM/IgA-enriched immunoglobulin preparation trimodulin on the complement pathway was investigated in in vitro opsonophagocytosis, binding and CDC assays. Immunoglobulin levels before and after trimodulin treatment were placed in relation to complement assessments in humans. In vitro, trimodulin activates complement and induces opsonophagocytosis, but also interacts with opsonins C3b, C4b and anaphylatoxin C5a in a concentration-dependent manner. This was not observed for standard intravenous IgG preparation (IVIg). Accordingly, trimodulin, but not IVIg, inhibited the downstream CDC pathway and target cell lysis. If applied at a similar concentration range in healthy subjects, trimodulin treatment resulted in C3 and C4 consumption in a concentration-dependent manner, which was extended in patients with severe community-acquired pneumonia. Complement consumption is found to be dependent on underlying immunoglobulin levels, particularly IgM, pinpointing their regulative function in humans. IgM/IgA provide a balancing effect on the complement system. Trimodulin may enhance phagocytosis and opsonophagocytosis in patients with severe infections and prevent excessive pathogen lysis and release of harmful anaphylatoxins.
Keywords: C4; CDC; IgA; IgG; IgM; anaphylatoxins; complement factors C3; immunomodulation; opsonophagocytosis; polyvalent immunoglobulin.
Conflict of interest statement
Authors are employees of Biotest AG, Dreieich, Germany. The authors declare that the research was conducted in the absence of any other commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
The Functional Role of IgA in the IgM/IgA-Enriched Immunoglobulin Preparation Trimodulin.Biomedicines. 2021 Dec 3;9(12):1828. doi: 10.3390/biomedicines9121828. Biomedicines. 2021. PMID: 34944644 Free PMC article.
-
The immunomodulating activity of trimodulin (polyvalent IgM, IgA, IgG solution): a post hoc analysis of the phase II CIGMA trial.Crit Care. 2023 Nov 9;27(1):436. doi: 10.1186/s13054-023-04719-9. Crit Care. 2023. PMID: 37946226 Free PMC article. Clinical Trial.
-
Efficacy and safety of trimodulin, a novel polyclonal antibody preparation, in patients with severe community-acquired pneumonia: a randomized, placebo-controlled, double-blind, multicenter, phase II trial (CIGMA study).Intensive Care Med. 2018 Apr;44(4):438-448. doi: 10.1007/s00134-018-5143-7. Epub 2018 Apr 9. Intensive Care Med. 2018. PMID: 29632995 Free PMC article. Clinical Trial.
-
Limited Innovations After More Than 65 Years of Immunoglobulin Replacement Therapy: Potential of IgA- and IgM-Enriched Formulations to Prevent Bacterial Respiratory Tract Infections.Front Immunol. 2018 Aug 23;9:1925. doi: 10.3389/fimmu.2018.01925. eCollection 2018. Front Immunol. 2018. PMID: 30190722 Free PMC article. Review.
-
Multifaceted Tissue-Protective Functions of Polyvalent Immunoglobulin Preparations in Severe Infections-Interactions with Neutrophils, Complement, and Coagulation Pathways.Biomedicines. 2023 Nov 10;11(11):3022. doi: 10.3390/biomedicines11113022. Biomedicines. 2023. PMID: 38002022 Free PMC article. Review.
Cited by
-
Blood-derived product therapies for SARS-CoV-2 infection and long COVID.MedComm (2020). 2023 Nov 15;4(6):e426. doi: 10.1002/mco2.426. eCollection 2023 Dec. MedComm (2020). 2023. PMID: 38020714 Free PMC article. Review.
-
Neonatal sepsis and transient immunodeficiency: Potential for novel immunoglobulin therapies?Front Immunol. 2022 Oct 18;13:1016877. doi: 10.3389/fimmu.2022.1016877. eCollection 2022. Front Immunol. 2022. PMID: 36330515 Free PMC article. Review.
-
Efficacy and safety of trimodulin in patients with severe COVID-19: results from a randomised, placebo-controlled, double-blind, multicentre, phase II trial (ESsCOVID).Eur J Med Res. 2024 Aug 13;29(1):418. doi: 10.1186/s40001-024-02008-x. Eur J Med Res. 2024. PMID: 39138518 Free PMC article. Clinical Trial.
-
The Functional Role of IgA in the IgM/IgA-Enriched Immunoglobulin Preparation Trimodulin.Biomedicines. 2021 Dec 3;9(12):1828. doi: 10.3390/biomedicines9121828. Biomedicines. 2021. PMID: 34944644 Free PMC article.
-
In Vitro Screening of Trehalose Synbiotics and Their Effects on Early-Lactating Females and Offspring Mice.Antioxidants (Basel). 2024 Oct 11;13(10):1223. doi: 10.3390/antiox13101223. Antioxidants (Basel). 2024. PMID: 39456476 Free PMC article.
References
-
- Janeway C., Travers P., Walport M., Shlomchik M.J. Immunobiology: The Immune System in Health and Disease. Trends Immunol. 2001;21:201.
LinkOut - more resources
Full Text Sources
Miscellaneous

