CD8+ T Cell Exhaustion in Cancer
- PMID: 34354714
- PMCID: PMC8330547
- DOI: 10.3389/fimmu.2021.715234
CD8+ T Cell Exhaustion in Cancer
Abstract
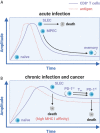
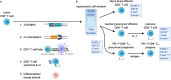
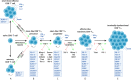
A paradigm shift in the understanding of the exhausted CD8+ T cell (Tex) lineage is underway. Originally thought to be a uniform population that progressively loses effector function in response to persistent antigen, single-cell analysis has now revealed that CD8+ Tex is composed of multiple interconnected subpopulations. The heterogeneity within the CD8+ Tex lineage is comprised of immune checkpoint blockade (ICB) permissive and refractory subsets termed stem-like and terminally differentiated cells, respectively. These populations occupy distinct peripheral and intratumoral niches and are characterized by transcriptional processes that govern transitions between cell states. This review presents key findings in the field to construct an updated view of the spatial, transcriptional, and functional heterogeneity of anti-tumoral CD8+ Tex. These emerging insights broadly call for (re-)focusing cancer immunotherapies to center on the driver mechanism(s) underlying the CD8+ Tex developmental continuum aimed at stabilizing functional subsets.
Keywords: CXCR3; PD-1/PD-L1; T cell exhaustion; T cell trafficking; cancer immunotherapy; co-stimulatory/inhibitory receptors; stem-like CD8+ T cells; tumor immunity.
Copyright © 2021 Dolina, Van Braeckel-Budimir, Thomas and Salek-Ardakani.
Conflict of interest statement
All authors are employees of Pfizer, Inc. and hold stock/stock options in the company.
Figures

Similar articles
-
Developmental Relationships of Four Exhausted CD8+ T Cell Subsets Reveals Underlying Transcriptional and Epigenetic Landscape Control Mechanisms.Immunity. 2020 May 19;52(5):825-841.e8. doi: 10.1016/j.immuni.2020.04.014. Epub 2020 May 11. Immunity. 2020. PMID: 32396847 Free PMC article.
-
CD8+ T cell exhaustion and its regulatory mechanisms in the tumor microenvironment: key to the success of immunotherapy.Front Immunol. 2024 Sep 20;15:1476904. doi: 10.3389/fimmu.2024.1476904. eCollection 2024. Front Immunol. 2024. PMID: 39372416 Free PMC article. Review.
-
Impact of CD4 T cells on intratumoral CD8 T-cell exhaustion and responsiveness to PD-1 blockade therapy in mouse brain tumors.J Immunother Cancer. 2022 Dec;10(12):e005293. doi: 10.1136/jitc-2022-005293. J Immunother Cancer. 2022. PMID: 36543376 Free PMC article.
-
TCF-1-Centered Transcriptional Network Drives an Effector versus Exhausted CD8 T Cell-Fate Decision.Immunity. 2019 Nov 19;51(5):840-855.e5. doi: 10.1016/j.immuni.2019.09.013. Epub 2019 Oct 9. Immunity. 2019. PMID: 31606264 Free PMC article.
-
Use of Mass Cytometry to Profile Human T Cell Exhaustion.Front Immunol. 2020 Jan 22;10:3039. doi: 10.3389/fimmu.2019.03039. eCollection 2019. Front Immunol. 2020. PMID: 32038613 Free PMC article. Review.
Cited by
-
CD8 + T cell-based molecular subtypes with heterogeneous immune landscapes and clinical significance in acute myeloid leukemia.Inflamm Res. 2024 Mar;73(3):329-344. doi: 10.1007/s00011-023-01839-4. Epub 2024 Jan 10. Inflamm Res. 2024. PMID: 38195768
-
Transcriptional regulation of DLGAP5 by AR suppresses p53 signaling and inhibits CD8+T cell infiltration in triple-negative breast cancer.Transl Oncol. 2024 Nov;49:102081. doi: 10.1016/j.tranon.2024.102081. Epub 2024 Aug 24. Transl Oncol. 2024. PMID: 39182361 Free PMC article.
-
Extensive-stage small-cell lung cancer in patients receiving atezolizumab plus carboplatin-etoposide: stratification of outcome based on a composite score that combines gene expression profiling and immune characterization of microenvironment.J Immunother Cancer. 2024 Jul 1;12(7):e008974. doi: 10.1136/jitc-2024-008974. J Immunother Cancer. 2024. PMID: 38955418 Free PMC article.
-
Lysine-Specific Demethylase 1 (LSD1)-Mediated Epigenetic Modification of Immunogenicity and Immunomodulatory Effects in Breast Cancers.Curr Oncol. 2023 Feb 9;30(2):2127-2143. doi: 10.3390/curroncol30020164. Curr Oncol. 2023. PMID: 36826125 Free PMC article. Review.
-
Construction and validation of chemoresistance-associated tumor- infiltrating exhausted-like CD8+ T cell signature in breast cancer: cr-TILCD8TSig.Front Immunol. 2023 Mar 6;14:1120886. doi: 10.3389/fimmu.2023.1120886. eCollection 2023. Front Immunol. 2023. PMID: 36949939 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

