MicroRNAs 21 and 199a-3p Regulate Axon Growth Potential through Modulation of Pten and mTo r mRNAs
- PMID: 34326064
- PMCID: PMC8362682
- DOI: 10.1523/ENEURO.0155-21.2021
MicroRNAs 21 and 199a-3p Regulate Axon Growth Potential through Modulation of Pten and mTo r mRNAs
Abstract
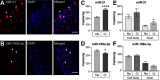
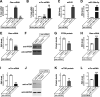
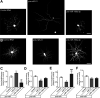
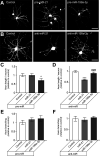
Increased mTOR activity has been shown to enhance regeneration of injured axons by increasing neuronal protein synthesis, while PTEN signaling can block mTOR activity to attenuate protein synthesis. MicroRNAs (miRs) have been implicated in regulation of PTEN and mTOR expression, and previous work in spinal cord showed an increase in miR-199a-3p after spinal cord injury (SCI) and increase in miR-21 in SCI animals that had undergone exercise. Pten mRNA is a target for miR-21 and miR-199a-3p is predicted to target mTor mRNA. Here, we show that miR-21 and miR-199a-3p are expressed in adult dorsal root ganglion (DRG) neurons, and we used culture preparations to test functions of the rat miRs in adult DRG and embryonic cortical neurons. miR-21 increases and miR-199a-3p decreases in DRG neurons after in vivo axotomy. In both the adult DRG and embryonic cortical neurons, miR-21 promotes and miR-199a-3p attenuates neurite growth. miR-21 directly bound to Pten mRNA and miR-21 overexpression decreased Pten mRNA levels. Conversely, miR-199a-3p directly bound to mTor mRNA and miR-199a-3p overexpression decreased mTor mRNA levels. Overexpressing miR-21 increased both overall and intra-axonal protein synthesis in cultured DRGs, while miR-199a-3p overexpression decreased this protein synthesis. The axon growth phenotypes seen with miR-21 and miR-199a-3p overexpression were reversed by co-transfecting PTEN and mTOR cDNA expression constructs with the predicted 3' untranslated region (UTR) miR target sequences deleted. Taken together, these studies indicate that injury-induced alterations in miR-21 and miR-199a-3p expression can alter axon growth capacity by changing overall and intra-axonal protein synthesis through regulation of the PTEN/mTOR pathway.
Keywords: PTEN; axonal mRNA; axonal translation; microRNA; regeneration; translation.
Copyright © 2021 Kar et al.
Figures




Similar articles
-
MiR-212-3p improves rat functional recovery and inhibits neurocyte apoptosis in spinal cord injury models via PTEN downregulation-mediated activation of AKT/mTOR pathway.Brain Res. 2021 Oct 1;1768:147576. doi: 10.1016/j.brainres.2021.147576. Epub 2021 Jul 1. Brain Res. 2021. PMID: 34216580
-
Exercise modulates microRNAs that affect the PTEN/mTOR pathway in rats after spinal cord injury.Exp Neurol. 2012 Jan;233(1):447-56. doi: 10.1016/j.expneurol.2011.11.018. Epub 2011 Nov 19. Exp Neurol. 2012. PMID: 22123082 Free PMC article.
-
miR-199a-3p is involved in estrogen-mediated autophagy through the IGF-1/mTOR pathway in osteocyte-like MLO-Y4 cells.J Cell Physiol. 2018 Mar;233(3):2292-2303. doi: 10.1002/jcp.26101. Epub 2017 Aug 25. J Cell Physiol. 2018. PMID: 28708244
-
Promoting axonal regeneration through exosomes: An update of recent findings on exosomal PTEN and mTOR modifiers.Brain Res Bull. 2018 Oct;143:123-131. doi: 10.1016/j.brainresbull.2018.10.008. Epub 2018 Oct 24. Brain Res Bull. 2018. PMID: 30367896 Review.
-
Axonal protein synthesis and the regulation of local mitochondrial function.Results Probl Cell Differ. 2009;48:225-42. doi: 10.1007/400_2009_1. Results Probl Cell Differ. 2009. PMID: 19343315 Free PMC article. Review.
Cited by
-
Dorsal Root Injury-A Model for Exploring Pathophysiology and Therapeutic Strategies in Spinal Cord Injury.Cells. 2021 Aug 25;10(9):2185. doi: 10.3390/cells10092185. Cells. 2021. PMID: 34571835 Free PMC article. Review.
-
Molecular mechanisms of exercise contributing to tissue regeneration.Signal Transduct Target Ther. 2022 Nov 30;7(1):383. doi: 10.1038/s41392-022-01233-2. Signal Transduct Target Ther. 2022. PMID: 36446784 Free PMC article. Review.
-
Newly discovered functions of miRNAs in neuropathic pain: Transitioning from recent discoveries to innovative underlying mechanisms.Mol Pain. 2024 Jan-Dec;20:17448069231225845. doi: 10.1177/17448069231225845. Mol Pain. 2024. PMID: 38148597 Free PMC article. Review.
-
MiRNAs as Promising Translational Strategies for Neuronal Repair and Regeneration in Spinal Cord Injury.Cells. 2022 Jul 12;11(14):2177. doi: 10.3390/cells11142177. Cells. 2022. PMID: 35883621 Free PMC article. Review.
-
Advances in molecular therapies for targeting pathophysiology in spinal cord injury.Expert Opin Ther Targets. 2023 Mar;27(3):171-187. doi: 10.1080/14728222.2023.2194532. Epub 2023 Apr 5. Expert Opin Ther Targets. 2023. PMID: 37017093 Free PMC article. Review.
References
-
- Al-Ali H, Ding Y, Slepak T, Wu W, Sun Y, Martinez Y, Xu XM, Lemmon VP, Bixby JL (2017) The mTOR substrate S6 kinase 1 (S6K1) is a negative regulator of axon regeneration and a potential drug target for central nervous system injury. J Neurosci 37:7079–7095. 10.1523/JNEUROSCI.0931-17.2017 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
