Outcomes of SARS-CoV-2 Infection in Patients With Chronic Liver Disease and Cirrhosis: A National COVID Cohort Collaborative Study
- PMID: 34284037
- PMCID: PMC8286237
- DOI: 10.1053/j.gastro.2021.07.010
Outcomes of SARS-CoV-2 Infection in Patients With Chronic Liver Disease and Cirrhosis: A National COVID Cohort Collaborative Study
Abstract
Background & aims: In patients with chronic liver disease (CLD) with or without cirrhosis, existing studies on the outcomes with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection have limited generalizability. We used the National COVID Cohort Collaborative (N3C), a harmonized electronic health record dataset of 6.4 million, to describe SARS-CoV-2 outcomes in patients with CLD and cirrhosis.
Methods: We identified all patients with CLD with or without cirrhosis who had SARS-CoV-2 testing in the N3C Data Enclave as of July 1, 2021. We used survival analyses to associate SARS-CoV-2 infection, presence of cirrhosis, and clinical factors with the primary outcome of 30-day mortality.
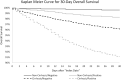
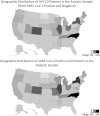
Results: We isolated 220,727 patients with CLD and SARS-CoV-2 test status: 128,864 (58%) were noncirrhosis/negative, 29,446 (13%) were noncirrhosis/positive, 53,476 (24%) were cirrhosis/negative, and 8941 (4%) were cirrhosis/positive patients. Thirty-day all-cause mortality rates were 3.9% in cirrhosis/negative and 8.9% in cirrhosis/positive patients. Compared to cirrhosis/negative patients, cirrhosis/positive patients had 2.38 times adjusted hazard of death at 30 days. Compared to noncirrhosis/positive patients, cirrhosis/positive patients had 3.31 times adjusted hazard of death at 30 days. In stratified analyses among patients with cirrhosis with increased age, obesity, and comorbid conditions (ie, diabetes, heart failure, and pulmonary disease), SARS-CoV-2 infection was associated with increased adjusted hazard of death.
Conclusions: In this study of approximately 221,000 nationally representative, diverse, and sex-balanced patients with CLD; we found SARS-CoV-2 infection in patients with cirrhosis was associated with 2.38 times mortality hazard, and the presence of cirrhosis among patients with CLD infected with SARS-CoV-2 was associated with 3.31 times mortality hazard. These results provide an additional impetus for increasing vaccination uptake and further research regarding immune responses to vaccines in patients with severe liver disease.
Keywords: COVID-19; Cirrhosis; N3C; OMOP; SARS-CoV-2.
Copyright © 2021 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures
Update of
-
Outcomes of SARS-CoV-2 Infection in Patients with Chronic Liver Disease and Cirrhosis: a N3C Study.medRxiv [Preprint]. 2021 Jun 7:2021.06.03.21258312. doi: 10.1101/2021.06.03.21258312. medRxiv. 2021. Update in: Gastroenterology. 2021 Nov;161(5):1487-1501.e5. doi: 10.1053/j.gastro.2021.07.010 PMID: 34127981 Free PMC article. Updated. Preprint.
Comment in
-
COVID-19 and Cirrhosis: A Combination We Must Strive to Prevent.Gastroenterology. 2021 Nov;161(5):1371-1373. doi: 10.1053/j.gastro.2021.08.037. Epub 2021 Aug 25. Gastroenterology. 2021. PMID: 34453892 Free PMC article. No abstract available.
-
COVID-19 and Social Determinants of Health in Gastroenterology and Hepatology.Gastroenterology. 2021 Nov;161(5):1373-1376. doi: 10.1053/j.gastro.2021.08.040. Epub 2021 Aug 26. Gastroenterology. 2021. PMID: 34454917 Free PMC article. No abstract available.
Similar articles
-
Outcomes of SARS-CoV-2 Infection in Patients with Chronic Liver Disease and Cirrhosis: a N3C Study.medRxiv [Preprint]. 2021 Jun 7:2021.06.03.21258312. doi: 10.1101/2021.06.03.21258312. medRxiv. 2021. Update in: Gastroenterology. 2021 Nov;161(5):1487-1501.e5. doi: 10.1053/j.gastro.2021.07.010 PMID: 34127981 Free PMC article. Updated. Preprint.
-
Breakthrough SARS-CoV-2 infection outcomes in vaccinated patients with chronic liver disease and cirrhosis: A National COVID Cohort Collaborative study.Hepatology. 2023 Mar 1;77(3):834-850. doi: 10.1002/hep.32780. Epub 2023 Feb 17. Hepatology. 2023. PMID: 36799617 Free PMC article.
-
Breakthrough SARS-CoV-2 Infection Outcomes in Vaccinated Patients with Chronic Liver Disease and Cirrhosis: A National COVID Cohort Collaborative Study.medRxiv [Preprint]. 2022 Jul 8:2022.02.25.22271490. doi: 10.1101/2022.02.25.22271490. medRxiv. 2022. PMID: 35821984 Free PMC article. Updated. Preprint.
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD013652. doi: 10.1002/14651858.CD013652.pub2. Cochrane Database Syst Rev. 2022. PMID: 36394900 Free PMC article. Review.
-
Liver fibrosis stage based on the four factors (FIB-4) score or Forns index in adults with chronic hepatitis C.Cochrane Database Syst Rev. 2024 Aug 13;8(8):CD011929. doi: 10.1002/14651858.CD011929.pub2. Cochrane Database Syst Rev. 2024. PMID: 39136280 Review.
Cited by
-
COVID-19-associated liver injury: Clinical characteristics, pathophysiological mechanisms and treatment management.Biomed Pharmacother. 2022 Oct;154:113568. doi: 10.1016/j.biopha.2022.113568. Epub 2022 Aug 17. Biomed Pharmacother. 2022. PMID: 36029543 Free PMC article. Review.
-
Liver and Biliary Tract Disease in Patients with Coronavirus disease-2019 Infection.Gastroenterol Clin North Am. 2023 Mar;52(1):13-36. doi: 10.1016/j.gtc.2022.09.001. Epub 2022 Oct 4. Gastroenterol Clin North Am. 2023. PMID: 36813421 Free PMC article. Review.
-
Real-world effectiveness of COVID-19 vaccination in liver cirrhosis: a systematic review with meta-analysis of 51,834 patients.Proc (Bayl Univ Med Cent). 2023 Jan 17;36(2):151-156. doi: 10.1080/08998280.2023.2165344. eCollection 2023. Proc (Bayl Univ Med Cent). 2023. PMID: 36876272 Free PMC article.
-
COVID-19 vaccines in patients with decompensated cirrhosis: a retrospective cohort on safety data and risk factors associated with unvaccinated status.Infect Dis Poverty. 2022 May 16;11(1):56. doi: 10.1186/s40249-022-00982-0. Infect Dis Poverty. 2022. PMID: 35578350 Free PMC article.
-
Clinical and Histopathological Discoveries in Patients with Hepatic Injury and Cholangiopathy Who Have Died of COVID-19: Insights and Opportunities for Intervention.Hepat Med. 2023 Oct 4;15:151-164. doi: 10.2147/HMER.S385133. eCollection 2023. Hepat Med. 2023. PMID: 37814605 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
- U54 GM104938/GM/NIGMS NIH HHS/United States
- UL1 TR002649/TR/NCATS NIH HHS/United States
- UL1 TR003167/TR/NCATS NIH HHS/United States
- UL1 TR001422/TR/NCATS NIH HHS/United States
- UL1 TR001860/TR/NCATS NIH HHS/United States
- U54 GM104942/GM/NIGMS NIH HHS/United States
- UL1 TR001420/TR/NCATS NIH HHS/United States
- UL1 TR001439/TR/NCATS NIH HHS/United States
- UL1 TR002243/TR/NCATS NIH HHS/United States
- UL1 TR001445/TR/NCATS NIH HHS/United States
- UL1 TR003096/TR/NCATS NIH HHS/United States
- UL1 TR002537/TR/NCATS NIH HHS/United States
- UL1 TR001412/TR/NCATS NIH HHS/United States
- UL1 TR001872/TR/NCATS NIH HHS/United States
- UL1 TR001878/TR/NCATS NIH HHS/United States
- UL1 TR002529/TR/NCATS NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- UL1 TR002494/TR/NCATS NIH HHS/United States
- P30 DK026743/DK/NIDDK NIH HHS/United States
- UL1 TR002736/TR/NCATS NIH HHS/United States
- U54 GM115516/GM/NIGMS NIH HHS/United States
- UL1 TR002369/TR/NCATS NIH HHS/United States
- UL1 TR002541/TR/NCATS NIH HHS/United States
- UL1 TR002001/TR/NCATS NIH HHS/United States
- UL1 TR002538/TR/NCATS NIH HHS/United States
- U54 GM115458/GM/NIGMS NIH HHS/United States
- UL1 TR001442/TR/NCATS NIH HHS/United States
- UL1 TR002535/TR/NCATS NIH HHS/United States
- UL1 TR001866/TR/NCATS NIH HHS/United States
- UL1 TR001449/TR/NCATS NIH HHS/United States
- UL1 TR001453/TR/NCATS NIH HHS/United States
- UL1 TR002489/TR/NCATS NIH HHS/United States
- U54 GM104940/GM/NIGMS NIH HHS/United States
- UL1 TR003107/TR/NCATS NIH HHS/United States
- UL1 TR003015/TR/NCATS NIH HHS/United States
- UL1 TR002733/TR/NCATS NIH HHS/United States
- UL1 TR001433/TR/NCATS NIH HHS/United States
- T32 DK060414/DK/NIDDK NIH HHS/United States
- U24 TR002306/TR/NCATS NIH HHS/United States
- UL1 TR002003/TR/NCATS NIH HHS/United States
- UL1 TR001876/TR/NCATS NIH HHS/United States
- UL1 TR001436/TR/NCATS NIH HHS/United States
- UL1 TR002378/TR/NCATS NIH HHS/United States
- UL1 TR002384/TR/NCATS NIH HHS/United States
- UL1 TR002553/TR/NCATS NIH HHS/United States
- UL1 TR002389/TR/NCATS NIH HHS/United States
- UL1 TR001414/TR/NCATS NIH HHS/United States
- U54 GM104941/GM/NIGMS NIH HHS/United States
- UL1 TR002014/TR/NCATS NIH HHS/United States
- UL1 TR002550/TR/NCATS NIH HHS/United States
- UL1 TR002319/TR/NCATS NIH HHS/United States
- R01 AG059183/AG/NIA NIH HHS/United States
- UL1 TR001855/TR/NCATS NIH HHS/United States
- UL1 TR001425/TR/NCATS NIH HHS/United States
- UL1 TR002373/TR/NCATS NIH HHS/United States
- UL1 TR002240/TR/NCATS NIH HHS/United States
- UL1 TR002556/TR/NCATS NIH HHS/United States
- UL1 TR003017/TR/NCATS NIH HHS/United States
- UL1 TR001998/TR/NCATS NIH HHS/United States
- UL1 TR001873/TR/NCATS NIH HHS/United States
- UL1 TR001881/TR/NCATS NIH HHS/United States
- UL1 TR002645/TR/NCATS NIH HHS/United States
- UL1 TR001450/TR/NCATS NIH HHS/United States
- UL1 TR002366/TR/NCATS NIH HHS/United States
- U54 GM115428/GM/NIGMS NIH HHS/United States
- UL1 TR002345/TR/NCATS NIH HHS/United States
- UL1 TR002377/TR/NCATS NIH HHS/United States
- U54 GM115677/GM/NIGMS NIH HHS/United States
- UL1 TR002544/TR/NCATS NIH HHS/United States
- UL1 TR003098/TR/NCATS NIH HHS/United States
- UL1 TR001430/TR/NCATS NIH HHS/United States
- UL1 TR003142/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous