Astrocytic interleukin-3 programs microglia and limits Alzheimer's disease
- PMID: 34262178
- PMCID: PMC8934148
- DOI: 10.1038/s41586-021-03734-6
Astrocytic interleukin-3 programs microglia and limits Alzheimer's disease
Abstract
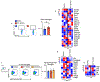
Communication within the glial cell ecosystem is essential for neuronal and brain health1-3. The influence of glial cells on the accumulation and clearance of β-amyloid (Aβ) and neurofibrillary tau in the brains of individuals with Alzheimer's disease (AD) is poorly understood, despite growing awareness that these are therapeutically important interactions4,5. Here we show, in humans and mice, that astrocyte-sourced interleukin-3 (IL-3) programs microglia to ameliorate the pathology of AD. Upon recognition of Aβ deposits, microglia increase their expression of IL-3Rα-the specific receptor for IL-3 (also known as CD123)-making them responsive to IL-3. Astrocytes constitutively produce IL-3, which elicits transcriptional, morphological, and functional programming of microglia to endow them with an acute immune response program, enhanced motility, and the capacity to cluster and clear aggregates of Aβ and tau. These changes restrict AD pathology and cognitive decline. Our findings identify IL-3 as a key mediator of astrocyte-microglia cross-talk and a node for therapeutic intervention in AD.
© 2021. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing interests
C.S.M., F.K.S., and R.E.T. are inventors on a patent application filed by Mass General Brigham that describes targeting IL-3 signaling in Alzheimer’s disease (invention record no. 2020-568). B.P.K. is an inventor on patent applications filed by Mass General Brigham that describe genome engineering technologies and methods, is an advisor to Acrigen Biosciences, and consults for Avectas Inc. and ElevateBio.
Figures





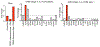
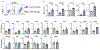
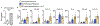



Comment in
-
Astrocytic IL-3 could help microglia protect against Alzheimer disease.Nat Rev Neurol. 2021 Sep;17(9):525. doi: 10.1038/s41582-021-00546-0. Nat Rev Neurol. 2021. PMID: 34321636 No abstract available.
Similar articles
-
Crosstalk between astrocytes and microglia results in increased degradation of α-synuclein and amyloid-β aggregates.J Neuroinflammation. 2021 Jun 3;18(1):124. doi: 10.1186/s12974-021-02158-3. J Neuroinflammation. 2021. PMID: 34082772 Free PMC article.
-
Interleukin-3 is associated with sTREM2 and mediates the correlation between amyloid-β and tau pathology in Alzheimer's disease.J Neuroinflammation. 2022 Dec 29;19(1):316. doi: 10.1186/s12974-022-02679-5. J Neuroinflammation. 2022. PMID: 36578067 Free PMC article.
-
Astrocyte interleukin-3 preps microglia.Trends Immunol. 2021 Nov;42(11):937-939. doi: 10.1016/j.it.2021.09.008. Epub 2021 Oct 14. Trends Immunol. 2021. PMID: 34657802 Free PMC article.
-
Effects of CX3CR1 and Fractalkine Chemokines in Amyloid Beta Clearance and p-Tau Accumulation in Alzheimer's Disease (AD) Rodent Models: Is Fractalkine a Systemic Biomarker for AD?Curr Alzheimer Res. 2016;13(4):403-12. doi: 10.2174/1567205013666151116125714. Curr Alzheimer Res. 2016. PMID: 26567742 Review.
-
Glial cells in Alzheimer's disease: From neuropathological changes to therapeutic implications.Ageing Res Rev. 2022 Jun;78:101622. doi: 10.1016/j.arr.2022.101622. Epub 2022 Apr 12. Ageing Res Rev. 2022. PMID: 35427810 Review.
Cited by
-
VEGFD/VEGFR3 signaling contributes to the dysfunction of the astrocyte IL-3/microglia IL-3Rα cross-talk and drives neuroinflammation in mouse ischemic stroke.Acta Pharmacol Sin. 2025 Feb;46(2):292-307. doi: 10.1038/s41401-024-01405-6. Epub 2024 Oct 30. Acta Pharmacol Sin. 2025. PMID: 39478160
-
Saponin components in Polygala tenuifolia as potential candidate drugs for treating dementia.Front Pharmacol. 2024 Jul 10;15:1431894. doi: 10.3389/fphar.2024.1431894. eCollection 2024. Front Pharmacol. 2024. PMID: 39050746 Free PMC article. Review.
-
Gut Microbiota Mediates Neuroinflammation in Alzheimer's Disease: Unraveling Key Factors and Mechanistic Insights.Mol Neurobiol. 2025 Mar;62(3):3746-3763. doi: 10.1007/s12035-024-04513-w. Epub 2024 Sep 25. Mol Neurobiol. 2025. PMID: 39317889 Review.
-
Novel Development and Prospects in Pathogenesis, Diagnosis, and Therapy of Alzheimer's Disease.J Alzheimers Dis Rep. 2024 Feb 20;8(1):345-354. doi: 10.3233/ADR-230130. eCollection 2024. J Alzheimers Dis Rep. 2024. PMID: 38405339 Free PMC article. Review.
-
Targeting tau in Alzheimer's disease: from mechanisms to clinical therapy.Neural Regen Res. 2024 Jul 1;19(7):1489-1498. doi: 10.4103/1673-5374.385847. Epub 2023 Sep 22. Neural Regen Res. 2024. PMID: 38051891 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- R00 HL151750/HL/NHLBI NIH HHS/United States
- R00 CA218870/CA/NCI NIH HHS/United States
- R35 GM139598/GM/NIGMS NIH HHS/United States
- P01 HL131478/HL/NHLBI NIH HHS/United States
- R01 CA158534/CA/NCI NIH HHS/United States
- R35 HL135752/HL/NHLBI NIH HHS/United States
- K99 HL151750/HL/NHLBI NIH HHS/United States
- R35 HL139598/HL/NHLBI NIH HHS/United States
- R01 HL158534/HL/NHLBI NIH HHS/United States
- R13 CA135752/CA/NCI NIH HHS/United States
- F31 HL147364/HL/NHLBI NIH HHS/United States
- P01 HL142494/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

