Bamlanivimab plus Etesevimab in Mild or Moderate Covid-19
- PMID: 34260849
- PMCID: PMC8314785
- DOI: 10.1056/NEJMoa2102685
Bamlanivimab plus Etesevimab in Mild or Moderate Covid-19
Abstract
Background: Patients with underlying medical conditions are at increased risk for severe coronavirus disease 2019 (Covid-19). Whereas vaccine-derived immunity develops over time, neutralizing monoclonal-antibody treatment provides immediate, passive immunity and may limit disease progression and complications.
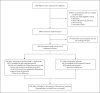
Methods: In this phase 3 trial, we randomly assigned, in a 1:1 ratio, a cohort of ambulatory patients with mild or moderate Covid-19 who were at high risk for progression to severe disease to receive a single intravenous infusion of either a neutralizing monoclonal-antibody combination agent (2800 mg of bamlanivimab and 2800 mg of etesevimab, administered together) or placebo within 3 days after a laboratory diagnosis of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. The primary outcome was the overall clinical status of the patients, defined as Covid-19-related hospitalization or death from any cause by day 29.
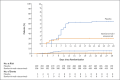
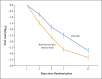
Results: A total of 1035 patients underwent randomization and received an infusion of bamlanivimab-etesevimab or placebo. The mean (±SD) age of the patients was 53.8±16.8 years, and 52.0% were adolescent girls or women. By day 29, a total of 11 of 518 patients (2.1%) in the bamlanivimab-etesevimab group had a Covid-19-related hospitalization or death from any cause, as compared with 36 of 517 patients (7.0%) in the placebo group (absolute risk difference, -4.8 percentage points; 95% confidence interval [CI], -7.4 to -2.3; relative risk difference, 70%; P<0.001). No deaths occurred in the bamlanivimab-etesevimab group; in the placebo group, 10 deaths occurred, 9 of which were designated by the trial investigators as Covid-19-related. At day 7, a greater reduction from baseline in the log viral load was observed among patients who received bamlanivimab plus etesevimab than among those who received placebo (difference from placebo in the change from baseline, -1.20; 95% CI, -1.46 to -0.94; P<0.001).
Conclusions: Among high-risk ambulatory patients, bamlanivimab plus etesevimab led to a lower incidence of Covid-19-related hospitalization and death than did placebo and accelerated the decline in the SARS-CoV-2 viral load. (Funded by Eli Lilly; BLAZE-1 ClinicalTrials.gov number, NCT04427501.).
Copyright © 2021 Massachusetts Medical Society.
Figures
Similar articles
-
Effect of Bamlanivimab as Monotherapy or in Combination With Etesevimab on Viral Load in Patients With Mild to Moderate COVID-19: A Randomized Clinical Trial.JAMA. 2021 Feb 16;325(7):632-644. doi: 10.1001/jama.2021.0202. JAMA. 2021. PMID: 33475701 Free PMC article. Clinical Trial.
-
A Randomized, Placebo-Controlled Clinical Trial of Bamlanivimab and Etesevimab Together in High-Risk Ambulatory Patients With COVID-19 and Validation of the Prognostic Value of Persistently High Viral Load.Clin Infect Dis. 2022 Aug 24;75(1):e440-e449. doi: 10.1093/cid/ciab912. Clin Infect Dis. 2022. PMID: 34718468 Free PMC article. Clinical Trial.
-
SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with Covid-19.N Engl J Med. 2021 Jan 21;384(3):229-237. doi: 10.1056/NEJMoa2029849. Epub 2020 Oct 28. N Engl J Med. 2021. PMID: 33113295 Free PMC article. Clinical Trial.
-
SARS-CoV-2-neutralising monoclonal antibodies for treatment of COVID-19.Cochrane Database Syst Rev. 2021 Sep 2;9(9):CD013825. doi: 10.1002/14651858.CD013825.pub2. Cochrane Database Syst Rev. 2021. PMID: 34473343 Free PMC article. Review.
-
A Narrative Review of the Clinical Practicalities of Bamlanivimab and Etesevimab Antibody Therapies for SARS-CoV-2.Infect Dis Ther. 2021 Dec;10(4):1933-1947. doi: 10.1007/s40121-021-00515-6. Epub 2021 Aug 10. Infect Dis Ther. 2021. PMID: 34374951 Free PMC article. Review.
Cited by
-
Fragment-based design of SARS-CoV-2 Mpro inhibitors.Struct Chem. 2022;33(6):2155-2168. doi: 10.1007/s11224-022-02031-w. Epub 2022 Aug 24. Struct Chem. 2022. PMID: 36035593 Free PMC article.
-
Recent review of COVID-19 management: diagnosis, treatment and vaccination.Pharmacol Rep. 2022 Dec;74(6):1120-1148. doi: 10.1007/s43440-022-00425-5. Epub 2022 Oct 10. Pharmacol Rep. 2022. PMID: 36214969 Free PMC article. Review.
-
The relationship between viral clearance rates and disease progression in early symptomatic COVID-19: a systematic review and meta-regression analysis.J Antimicrob Chemother. 2024 May 2;79(5):935-945. doi: 10.1093/jac/dkae045. J Antimicrob Chemother. 2024. PMID: 38385479 Free PMC article.
-
Absence of Mortality Differences Between the First and Second COVID-19 Waves in Kidney Transplant Recipients.Kidney Int Rep. 2022 Dec;7(12):2617-2629. doi: 10.1016/j.ekir.2022.09.007. Epub 2022 Sep 21. Kidney Int Rep. 2022. PMID: 36159445 Free PMC article.
-
Monoclonal antibodies against SARS-CoV-2 to prevent COVID-19 worsening in a large multicenter cohort.Heliyon. 2024 Aug 13;10(16):e36102. doi: 10.1016/j.heliyon.2024.e36102. eCollection 2024 Aug 30. Heliyon. 2024. PMID: 39247344 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
