mTORC2: The other mTOR in autophagy regulation
- PMID: 34250734
- PMCID: PMC8373318
- DOI: 10.1111/acel.13431
mTORC2: The other mTOR in autophagy regulation
Abstract
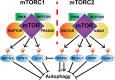
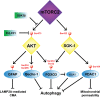
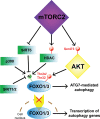
The mechanistic target of rapamycin (mTOR) has gathered significant attention as a ubiquitously expressed multimeric kinase with key implications for cell growth, proliferation, and survival. This kinase forms the central core of two distinct complexes, mTORC1 and mTORC2, which share the ability of integrating environmental, nutritional, and hormonal cues but which regulate separate molecular pathways that result in different cellular responses. Particularly, mTORC1 has been described as a major negative regulator of endosomal biogenesis and autophagy, a catabolic process that degrades intracellular components and organelles within the lysosomes and is thought to play a key role in human health and disease. In contrast, the role of mTORC2 in the regulation of autophagy has been considerably less studied despite mounting evidence this complex may regulate autophagy in a different and perhaps complementary manner to that of mTORC1. Genetic ablation of unique subunits is currently being utilized to study the differential effects of the two mTOR complexes. RICTOR is the best-described subunit specific to mTORC2 and as such has become a useful tool for investigating the specific actions of this complex. The development of complex-specific inhibitors for mTORC2 is also an area of intense interest. Studies to date have demonstrated that mTORC1/2 complexes each signal to a variety of exclusive downstream molecules with distinct biological roles. Pinpointing the particular effects of these downstream effectors is crucial toward the development of novel therapies aimed at accurately modulating autophagy in the context of human aging and disease.
Keywords: AKT; FOXOs; SGK-1; autophagy; mTORC2.
© 2021 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Similar articles
-
Diverse signaling mechanisms of mTOR complexes: mTORC1 and mTORC2 in forming a formidable relationship.Adv Biol Regul. 2019 May;72:51-62. doi: 10.1016/j.jbior.2019.03.003. Epub 2019 Apr 11. Adv Biol Regul. 2019. PMID: 31010692 Review.
-
mTORC2: a multifaceted regulator of autophagy.Cell Commun Signal. 2023 Jan 5;21(1):4. doi: 10.1186/s12964-022-00859-7. Cell Commun Signal. 2023. PMID: 36604720 Free PMC article. Review.
-
Discrete signaling mechanisms of mTORC1 and mTORC2: Connected yet apart in cellular and molecular aspects.Adv Biol Regul. 2017 May;64:39-48. doi: 10.1016/j.jbior.2016.12.001. Epub 2017 Jan 4. Adv Biol Regul. 2017. PMID: 28189457 Review.
-
mTORC2: A neglected player in aging regulation.J Cell Physiol. 2024 Nov;239(11):e31363. doi: 10.1002/jcp.31363. Epub 2024 Jul 10. J Cell Physiol. 2024. PMID: 38982866 Review.
-
TGFB-INHB/activin signaling regulates age-dependent autophagy and cardiac health through inhibition of MTORC2.Autophagy. 2020 Oct;16(10):1807-1822. doi: 10.1080/15548627.2019.1704117. Epub 2019 Dec 29. Autophagy. 2020. PMID: 31884871 Free PMC article.
Cited by
-
Traditional Chinese medicine and its active substances reduce vascular injury in diabetes via regulating autophagic activity.Front Pharmacol. 2024 Mar 4;15:1355246. doi: 10.3389/fphar.2024.1355246. eCollection 2024. Front Pharmacol. 2024. PMID: 38505420 Free PMC article. Review.
-
Diabetes and Osteoarthritis: Exploring the Interactions and Therapeutic Implications of Insulin, Metformin, and GLP-1-Based Interventions.Biomedicines. 2024 Jul 23;12(8):1630. doi: 10.3390/biomedicines12081630. Biomedicines. 2024. PMID: 39200096 Free PMC article. Review.
-
Nutrition Strategies Promoting Healthy Aging: From Improvement of Cardiovascular and Brain Health to Prevention of Age-Associated Diseases.Nutrients. 2022 Dec 22;15(1):47. doi: 10.3390/nu15010047. Nutrients. 2022. PMID: 36615705 Free PMC article. Review.
-
Repurposing autophagy regulators in brain tumors.Int J Cancer. 2022 Jul 15;151(2):167-180. doi: 10.1002/ijc.33965. Epub 2022 Mar 4. Int J Cancer. 2022. PMID: 35179776 Free PMC article. Review.
-
Fatty Acids as Potent Modulators of Autophagy Activity in White Adipose Tissue.Biomolecules. 2023 Jan 30;13(2):255. doi: 10.3390/biom13020255. Biomolecules. 2023. PMID: 36830623 Free PMC article. Review.
References
-
- Amaravadi, R. K., Lippincott‐Schwartz, J., Yin, X.‐M., Weiss, W. A., Takebe, N., Timmer, W., DiPaola, R. S., Lotze, M. T., & White, E. (2011). Principles and current strategies for targeting autophagy for cancer treatment. Clinical Cancer Research, 17(4), 654–666. 10.1158/1078-0432.CCR-10-2634 - DOI - PMC - PubMed
-
- Arriola Apelo, S. I., Lin, A., Brinkman, J. A., Meyer, E., Morrison, M., Tomasiewicz, J. L., Pumper, C. P., Baar, E. L., Richardson, N. E., Alotaibi, M., & Lamming, D. W. (2020). Ovariectomy uncouples lifespan from metabolic health and reveals a sex‐hormone‐dependent role of hepatic mTORC2 in aging. eLife, 9, e56177. 10.7554/elife.56177 - DOI - PMC - PubMed
-
- Arriola Apelo, S. I., Neuman, J. C., Baar, E. L., Syed, F. A., Cummings, N. E., Brar, H. K., Pumper, C. P., Kimple, M. E., & Lamming, D. W. (2016). Alternative rapamycin treatment regimens mitigate the impact of rapamycin on glucose homeostasis and the immune system. Aging Cell, 15(1), 28–38. 10.1111/acel.12405 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

