Overview of Evidence-Based Chemotherapy for Oral Cancer: Focus on Drug Resistance Related to the Epithelial-Mesenchymal Transition
- PMID: 34208465
- PMCID: PMC8234904
- DOI: 10.3390/biom11060893
Overview of Evidence-Based Chemotherapy for Oral Cancer: Focus on Drug Resistance Related to the Epithelial-Mesenchymal Transition
Abstract
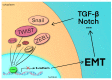
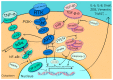
The increasing incidence of resistance to chemotherapeutic agents has become a major issue in the treatment of oral cancer (OC). Epithelial-mesenchymal transition (EMT) has attracted a great deal of attention in recent years with regard to its relation to the mechanism of chemotherapy drug resistance. EMT-activating transcription factors (EMT-ATFs), such as Snail, TWIST, and ZEB, can activate several different molecular pathways, e.g., PI3K/AKT, NF-κB, and TGF-β. In contrast, the activated oncological signal pathways provide reciprocal feedback that affects the expression of EMT-ATFs, resulting in a peritumoral extracellular environment conducive to cancer cell survival and evasion of the immune system, leading to resistance to multiple chemotherapeutic agents. We present an overview of evidence-based chemotherapy for OC treatment based on the National Comprehensive Cancer Network (NCCN) Chemotherapy Order Templates. We focus on the molecular pathways involved in drug resistance related to the EMT and highlight the signal pathways and transcription factors that may be important for EMT-regulated drug resistance. Rapid progress in antitumor regimens, together with the application of powerful techniques such as high-throughput screening and microRNA technology, will facilitate the development of therapeutic strategies to augment chemotherapy.
Keywords: EMT; chemoresistance; chemotherapy; oral squamous cell carcinoma.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures

Similar articles
-
Association of the Epithelial-Mesenchymal Transition (EMT) with Cisplatin Resistance.Int J Mol Sci. 2020 Jun 3;21(11):4002. doi: 10.3390/ijms21114002. Int J Mol Sci. 2020. PMID: 32503307 Free PMC article. Review.
-
Acquisition of epithelial-mesenchymal transition phenotype and cancer stem cell-like properties in cisplatin-resistant lung cancer cells through AKT/β-catenin/Snail signaling pathway.Eur J Pharmacol. 2014 Jan 15;723:156-66. doi: 10.1016/j.ejphar.2013.12.004. Epub 2013 Dec 12. Eur J Pharmacol. 2014. PMID: 24333218
-
Emodin: One Main Ingredient of Shufeng Jiedu Capsule Reverses Chemoresistance of Lung Cancer Cells Through Inhibition of EMT.Cell Physiol Biochem. 2017;42(3):1063-1072. doi: 10.1159/000478754. Epub 2017 Jun 28. Cell Physiol Biochem. 2017. PMID: 28662514
-
p73 induction by Abrus agglutinin facilitates Snail ubiquitination to inhibit epithelial to mesenchymal transition in oral cancer.Phytomedicine. 2019 Mar 1;55:179-190. doi: 10.1016/j.phymed.2018.08.003. Epub 2018 Aug 6. Phytomedicine. 2019. PMID: 30668428
-
Epithelial-mesenchymal-transition-inducing transcription factors: new targets for tackling chemoresistance in cancer?Oncogene. 2018 Nov;37(48):6195-6211. doi: 10.1038/s41388-018-0378-x. Epub 2018 Jul 12. Oncogene. 2018. PMID: 30002444 Review.
Cited by
-
Eugenol as a potential adjuvant therapy for gingival squamous cell carcinoma.Sci Rep. 2024 May 13;14(1):10958. doi: 10.1038/s41598-024-60754-8. Sci Rep. 2024. PMID: 38740853 Free PMC article.
-
Five EMT-Related Gene Signatures Predict Acute Myeloid Leukemia Patient Outcome.Dis Markers. 2022 Oct 7;2022:7826393. doi: 10.1155/2022/7826393. eCollection 2022. Dis Markers. 2022. PMID: 36246561 Free PMC article.
-
Transthyretin promotes the invasion of combined hepatocellular cholangiocarcinoma by tumor-associated macrophages.Cancer Rep (Hoboken). 2023 Oct;6(10):e1888. doi: 10.1002/cnr2.1888. Epub 2023 Sep 9. Cancer Rep (Hoboken). 2023. PMID: 37688511 Free PMC article.
-
The Epithelial-Mesenchymal Transition Influences the Resistance of Oral Squamous Cell Carcinoma to Monoclonal Antibodies via Its Effect on Energy Homeostasis and the Tumor Microenvironment.Cancers (Basel). 2021 Nov 24;13(23):5905. doi: 10.3390/cancers13235905. Cancers (Basel). 2021. PMID: 34885013 Free PMC article. Review.
-
Determination of the anticancer activity of standardized extract of Centella asiatica (ECa 233) on cell growth and metastatic behavior in oral cancer cells.Res Pharm Sci. 2024 Apr 1;19(2):121-147. doi: 10.4103/RPS.RPS_81_23. eCollection 2024 Apr. Res Pharm Sci. 2024. PMID: 39035578 Free PMC article.
References
-
- Muwonge R., Ramadas K., Sankila R., Thara S., Thomas G., Vinoda J., Sankaranarayanan R. Role of tobacco smoking, chewing and alcohol drinking in the risk of oral cancer in Trivandrum, India: A nested case-control design using incident cancer cases. Oral Oncol. 2008;44:446–454. doi: 10.1016/j.oraloncology.2007.06.002. - DOI - PubMed
-
- Martinez-Trufero J., Isla D., Adansa J.C., Irigoyen A., Hitt R., Gil-Arnaiz I., Lambea J., Lecumberri M.J., Cruz J.J. Phase II study of capecitabine as palliative treatment for patients with recurrent and metastatic squamous head and neck cancer after previous platinum-based treatment. Br. J. Cancer. 2010;102:1687–1691. doi: 10.1038/sj.bjc.6605697. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

