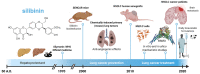
Lung Cancer Management with Silibinin: A Historical and Translational Perspective
- PMID: 34208282
- PMCID: PMC8230811
- DOI: 10.3390/ph14060559
Lung Cancer Management with Silibinin: A Historical and Translational Perspective
Abstract
The flavonolignan silibinin, the major bioactive component of the silymarin extract of Silybum marianum (milk thistle) seeds, is gaining traction as a novel anti-cancer therapeutic. Here, we review the historical developments that have laid the groundwork for the evaluation of silibinin as a chemopreventive and therapeutic agent in human lung cancer, including translational insights into its mechanism of action to control the aggressive behavior of lung carcinoma subtypes prone to metastasis. First, we summarize the evidence from chemically induced primary lung tumors supporting a role for silibinin in lung cancer prevention. Second, we reassess the preclinical and clinical evidence on the effectiveness of silibinin against drug resistance and brain metastasis traits of lung carcinomas. Third, we revisit the transcription factor STAT3 as a central tumor-cell intrinsic and microenvironmental target of silibinin in primary lung tumors and brain metastasis. Finally, by unraveling the selective vulnerability of silibinin-treated tumor cells to drugs using CRISPR-based chemosensitivity screenings (e.g., the hexosamine biosynthesis pathway inhibitor azaserine), we illustrate how the therapeutic use of silibinin against targetable weaknesses might be capitalized in specific lung cancer subtypes (e.g., KRAS/STK11 co-mutant tumors). Forthcoming studies should take up the challenge of developing silibinin and/or next-generation silibinin derivatives as novel lung cancer-preventive and therapeutic biomolecules.
Keywords: EMT; STAT3; metastasis; non-small cell lung cancer; silibinin; silymarin.
Conflict of interest statement
Joaquim Bosch-Barrera reports grants and personal fees from Roche-Genentech, grants from Pfizer and Pierre Fabre, and personal fees from MSD, BMS, AstraZeneca, Boehringer-Ingelheim, and Novartis, outside the submitted work. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Silibinin meglumine, a water-soluble form of milk thistle silymarin, is an orally active anti-cancer agent that impedes the epithelial-to-mesenchymal transition (EMT) in EGFR-mutant non-small-cell lung carcinoma cells.Food Chem Toxicol. 2013 Oct;60:360-8. doi: 10.1016/j.fct.2013.07.063. Epub 2013 Aug 1. Food Chem Toxicol. 2013. PMID: 23916468
-
Targeting STAT3 with silibinin to improve cancer therapeutics.Cancer Treat Rev. 2017 Jul;58:61-69. doi: 10.1016/j.ctrv.2017.06.003. Epub 2017 Jun 23. Cancer Treat Rev. 2017. PMID: 28686955 Review.
-
Silibinin and STAT3: A natural way of targeting transcription factors for cancer therapy.Cancer Treat Rev. 2015 Jun;41(6):540-6. doi: 10.1016/j.ctrv.2015.04.008. Epub 2015 Apr 27. Cancer Treat Rev. 2015. PMID: 25944486 Review.
-
The flavonolignan silibinin potentiates TRAIL-induced apoptosis in human colon adenocarcinoma and in derived TRAIL-resistant metastatic cells.Apoptosis. 2012 Aug;17(8):797-809. doi: 10.1007/s10495-012-0731-4. Apoptosis. 2012. PMID: 22555452
-
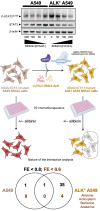
STAT3-targeted treatment with silibinin overcomes the acquired resistance to crizotinib in ALK-rearranged lung cancer.Cell Cycle. 2016 Dec 16;15(24):3413-3418. doi: 10.1080/15384101.2016.1245249. Epub 2016 Oct 18. Cell Cycle. 2016. PMID: 27753543 Free PMC article.
Cited by
-
Therapeutic Potential of Natural Resources Against Endometriosis: Current Advances and Future Perspectives.Drug Des Devel Ther. 2024 Aug 21;18:3667-3696. doi: 10.2147/DDDT.S464910. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 39188919 Free PMC article. Review.
-
Silibinin Induces Both Apoptosis and Necroptosis with Potential Anti-tumor Efficacy in Lung Cancer.Anticancer Agents Med Chem. 2024;24(18):1327-1338. doi: 10.2174/0118715206295371240724092314. Anticancer Agents Med Chem. 2024. PMID: 39069713
-
The Versatility of Collagen in Pharmacology: Targeting Collagen, Targeting with Collagen.Int J Mol Sci. 2024 Jun 13;25(12):6523. doi: 10.3390/ijms25126523. Int J Mol Sci. 2024. PMID: 38928229 Free PMC article. Review.
-
A comprehensive evaluation of the therapeutic potential of silibinin: a ray of hope in cancer treatment.Front Pharmacol. 2024 Feb 29;15:1349745. doi: 10.3389/fphar.2024.1349745. eCollection 2024. Front Pharmacol. 2024. PMID: 38487172 Free PMC article. Review.
-
Silibinin Overcomes EMT-Driven Lung Cancer Resistance to New-Generation ALK Inhibitors.Cancers (Basel). 2022 Dec 11;14(24):6101. doi: 10.3390/cancers14246101. Cancers (Basel). 2022. PMID: 36551587 Free PMC article.
References
-
- Middleton E., Jr., Kandaswami C., Theoharides T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000;52:673–751. - PubMed
-
- Barrajón-Catalán E., Herranz-López M., Joven J., Segura-Carretero A., Alonso-Villaverde C., Menéndez J.A., Micol V. Molecular promiscuity of plant polyphenols in the management of age-related diseases: Far beyond their antioxidant properties. Adv. Exp. Med. Biol. 2014;824:141–159. - PubMed
-
- Menendez J.A., Joven J., Aragonès G., Barrajón-Catalán E., Beltrán-Debón R., Borrás-Linares I., Camps J., Corominas-Faja B., Cufí S., Fernández-Arroyo S., et al. Xenohormetic and anti-aging activity of secoiridoid polyphenols present in extra virgin olive oil: A new family of gerosuppressant agents. Cell Cycle. 2013;12:555–578. doi: 10.4161/cc.23756. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

