Mechanistic basis for receptor-mediated pathological α-synuclein fibril cell-to-cell transmission in Parkinson's disease
- PMID: 34172566
- PMCID: PMC8256039
- DOI: 10.1073/pnas.2011196118
Mechanistic basis for receptor-mediated pathological α-synuclein fibril cell-to-cell transmission in Parkinson's disease
Abstract
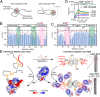
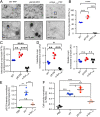
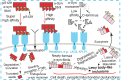
The spread of pathological α-synuclein (α-syn) is a crucial event in the progression of Parkinson's disease (PD). Cell surface receptors such as lymphocyte activation gene 3 (LAG3) and amyloid precursor-like protein 1 (APLP1) can preferentially bind α-syn in the amyloid over monomeric state to initiate cell-to-cell transmission. However, the molecular mechanism underlying this selective binding is unknown. Here, we perform an array of biophysical experiments and reveal that LAG3 D1 and APLP1 E1 domains commonly use an alkaline surface to bind the acidic C terminus, especially residues 118 to 140, of α-syn. The formation of amyloid fibrils not only can disrupt the intramolecular interactions between the C terminus and the amyloid-forming core of α-syn but can also condense the C terminus on fibril surface, which remarkably increase the binding affinity of α-syn to the receptors. Based on this mechanism, we find that phosphorylation at serine 129 (pS129), a hallmark modification of pathological α-syn, can further enhance the interaction between α-syn fibrils and the receptors. This finding is further confirmed by the higher efficiency of pS129 fibrils in cellular internalization, seeding, and inducing PD-like α-syn pathology in transgenic mice. Our work illuminates the mechanistic understanding on the spread of pathological α-syn and provides structural information for therapeutic targeting on the interaction of α-syn fibrils and receptors as a potential treatment for PD.
Keywords: Parkinson’s disease; cell-to-cell transmission; posttranslational modification; α-synuclein.
Conflict of interest statement
The authors declare no competing interest.
Figures





Similar articles
-
Aplp1 interacts with Lag3 to facilitate transmission of pathologic α-synuclein.Nat Commun. 2024 May 31;15(1):4663. doi: 10.1038/s41467-024-49016-3. Nat Commun. 2024. PMID: 38821932 Free PMC article.
-
Pathological α-synuclein transmission initiated by binding lymphocyte-activation gene 3.Science. 2016 Sep 30;353(6307):aah3374. doi: 10.1126/science.aah3374. Science. 2016. PMID: 27708076 Free PMC article.
-
Parkinson's disease-related phosphorylation at Tyr39 rearranges α-synuclein amyloid fibril structure revealed by cryo-EM.Proc Natl Acad Sci U S A. 2020 Aug 18;117(33):20305-20315. doi: 10.1073/pnas.1922741117. Epub 2020 Jul 31. Proc Natl Acad Sci U S A. 2020. PMID: 32737160 Free PMC article.
-
Alteration of Structure and Aggregation of α-Synuclein by Familial Parkinson's Disease Associated Mutations.Curr Protein Pept Sci. 2017;18(7):656-676. doi: 10.2174/1389203717666160314151706. Curr Protein Pept Sci. 2017. PMID: 26972727 Review.
-
Aggregation and phase separation of α-synuclein in Parkinson's disease.Chem Commun (Camb). 2024 Jun 25;60(52):6581-6590. doi: 10.1039/d4cc01591f. Chem Commun (Camb). 2024. PMID: 38808534 Review.
Cited by
-
Specific binding of Hsp27 and phosphorylated Tau mitigates abnormal Tau aggregation-induced pathology.Elife. 2022 Sep 1;11:e79898. doi: 10.7554/eLife.79898. Elife. 2022. PMID: 36048712 Free PMC article.
-
Targeting amyloid proteins for clinical diagnosis of neurodegenerative diseases.Fundam Res. 2022 Nov 1;3(4):505-519. doi: 10.1016/j.fmre.2022.10.009. eCollection 2023 Jul. Fundam Res. 2022. PMID: 38933553 Free PMC article. Review.
-
Seven mysteries of LAG-3: a multi-faceted immune receptor of increasing complexity.Immunother Adv. 2021 Dec 20;2(1):ltab025. doi: 10.1093/immadv/ltab025. eCollection 2022. Immunother Adv. 2021. PMID: 35265944 Free PMC article. Review.
-
Realization of Amyloid-like Aggregation as a Common Cause for Pathogenesis in Diseases.Life (Basel). 2023 Jul 7;13(7):1523. doi: 10.3390/life13071523. Life (Basel). 2023. PMID: 37511898 Free PMC article. Review.
-
Different charged biopolymers induce α-synuclein to form fibrils with distinct structures.J Biol Chem. 2024 Nov;300(11):107862. doi: 10.1016/j.jbc.2024.107862. Epub 2024 Oct 5. J Biol Chem. 2024. PMID: 39374778 Free PMC article.
References
-
- Riek R., Eisenberg D. S., The activities of amyloids from a structural perspective. Nature 539, 227–235 (2016). - PubMed
-
- Kordower J. H., Chu Y., Hauser R. A., Freeman T. B., Olanow C. W., Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat. Med. 14, 504–506 (2008). - PubMed
-
- Braak H., Sandmann-Keil D., Gai W., Braak E., Extensive axonal Lewy neurites in Parkinson’s disease: A novel pathological feature revealed by alpha-synuclein immunocytochemistry. Neurosci. Lett. 265, 67–69 (1999). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

