Early Convalescent Plasma Therapy and Mortality Among US Veterans Hospitalized With Nonsevere COVID-19: An Observational Analysis Emulating a Target Trial
- PMID: 34153099
- PMCID: PMC8411382
- DOI: 10.1093/infdis/jiab330
Early Convalescent Plasma Therapy and Mortality Among US Veterans Hospitalized With Nonsevere COVID-19: An Observational Analysis Emulating a Target Trial
Abstract
Background: Early convalescent plasma transfusion may reduce mortality in patients with nonsevere coronavirus disease 2019 (COVID-19).
Methods: This study emulates a (hypothetical) target trial using observational data from a cohort of US veterans admitted to a Department of Veterans Affairs (VA) facility between 1 May and 17 November 2020 with nonsevere COVID-19. The intervention was convalescent plasma initiated within 2 days of eligibility. Thirty-day mortality was compared using cumulative incidence curves, risk differences, and hazard ratios estimated from pooled logistic models with inverse probability weighting to adjust for confounding.
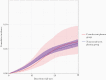
Results: Of 11 269 eligible person-trials contributed by 4755 patients, 402 trials were assigned to the convalescent plasma group. Forty and 671 deaths occurred within the plasma and nonplasma groups, respectively. The estimated 30-day mortality risk was 6.5% (95% confidence interval [CI], 4.0%-9.7%) in the plasma group and 6.2% (95% CI, 5.6%-7.0%) in the nonplasma group. The associated risk difference was 0.30% (95% CI, -2.30% to 3.60%) and the hazard ratio was 1.04 (95% CI, .64-1.62).
Conclusions: Our target trial emulation estimated no meaningful differences in 30-day mortality between nonsevere COVID-19 patients treated and untreated with convalescent plasma. Clinical Trials Registration. NCT04545047.
Keywords: COVID-19; SARS-CoV-2; convalescent plasma; coronavirus.
Published by Oxford University Press for the Infectious Diseases Society of America 2021.
Figures
Similar articles
-
Randomized clinical trial to evaluate safety and efficacy of convalescent plasma use among hospitalized patients with COVID-19 (PERUCONPLASMA): a structured summary of a study protocol for a randomized controlled trial.Trials. 2021 May 17;22(1):342. doi: 10.1186/s13063-021-05189-6. Trials. 2021. PMID: 34001174 Free PMC article.
-
A Randomized Trial of Convalescent Plasma in Covid-19 Severe Pneumonia.N Engl J Med. 2021 Feb 18;384(7):619-629. doi: 10.1056/NEJMoa2031304. Epub 2020 Nov 24. N Engl J Med. 2021. PMID: 33232588 Free PMC article. Clinical Trial.
-
Reconvalescent plasma/camostat mesylate in early SARS-CoV-2 Q-PCR positive high-risk individuals (RES-Q-HR): a structured summary of a study protocol for a randomized controlled trial.Trials. 2021 May 17;22(1):343. doi: 10.1186/s13063-021-05181-0. Trials. 2021. PMID: 34001215 Free PMC article.
-
[Transfusion of convalescent plasma from patients with COVID -19].Rev Peru Med Exp Salud Publica. 2020 Oct-Dec;37(4):746-754. doi: 10.17843/rpmesp.2020.374.5767. Epub 2021 Feb 3. Rev Peru Med Exp Salud Publica. 2020. PMID: 33566918 Review. Spanish.
-
Convalescent plasma therapy in COVID-19 critically ill patients during advanced phases of clinical trials and their preliminary results.Einstein (Sao Paulo). 2021 Apr 19;19:eRW6186. doi: 10.31744/einstein_journal/2021RW6186. eCollection 2021. Einstein (Sao Paulo). 2021. PMID: 33886937 Free PMC article. Review.
Cited by
-
The efficiency of convalescent plasma in COVID-19 patients: A systematic review and meta-analysis of randomized controlled clinical trials.Front Immunol. 2022 Jul 28;13:964398. doi: 10.3389/fimmu.2022.964398. eCollection 2022. Front Immunol. 2022. PMID: 35967398 Free PMC article.
-
COVID-19 in Veterans: A Narrative Review.Risk Manag Healthc Policy. 2022 Apr 26;15:805-815. doi: 10.2147/RMHP.S354814. eCollection 2022. Risk Manag Healthc Policy. 2022. PMID: 35502442 Free PMC article. Review.
-
Early administration of COVID-19 convalescent plasma with high titer antibody content by live viral neutralization assay is associated with modest clinical efficacy.Am J Hematol. 2022 Jun 1;97(6):770-779. doi: 10.1002/ajh.26531. Epub 2022 Mar 24. Am J Hematol. 2022. PMID: 35303377 Free PMC article.
-
CONVALESCENT plasma for COVID-19: A meta-analysis of clinical trials and real-world evidence.Eur J Clin Invest. 2021 Nov;51(11):e13663. doi: 10.1111/eci.13663. Epub 2021 Aug 18. Eur J Clin Invest. 2021. PMID: 34375445 Free PMC article.
-
Benefit and risk of oral anticoagulant initiation strategies in patients with atrial fibrillation and cancer: a target trial emulation using the SEER-Medicare database.J Thromb Thrombolysis. 2024 Apr;57(4):638-649. doi: 10.1007/s11239-024-02958-3. Epub 2024 Mar 20. J Thromb Thrombolysis. 2024. PMID: 38504063 Free PMC article.
References
-
- Sethuraman N, Jeremiah SS, Ryo A. Interpreting diagnostic tests for SARS-CoV-2. JAMA 2020; 323:2249–51. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

