Chemoenzymatic modular assembly of O-GalNAc glycans for functional glycomics
- PMID: 34117223
- PMCID: PMC8196059
- DOI: 10.1038/s41467-021-23428-x
Chemoenzymatic modular assembly of O-GalNAc glycans for functional glycomics
Abstract
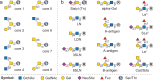
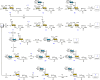
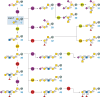
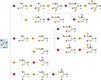
O-GalNAc glycans (or mucin O-glycans) play pivotal roles in diverse biological and pathological processes, including tumor growth and progression. Structurally defined O-GalNAc glycans are essential for functional studies but synthetic challenges and their inherent structural diversity and complexity have limited access to these compounds. Herein, we report an efficient and robust chemoenzymatic modular assembly (CEMA) strategy to construct structurally diverse O-GalNAc glycans. The key to this strategy is the convergent assembly of O-GalNAc cores 1-4 and 6 from three chemical building blocks, followed by enzymatic diversification of the cores by 13 well-tailored enzyme modules. A total of 83 O-GalNAc glycans presenting various natural glycan epitopes are obtained and used to generate a unique synthetic mucin O-glycan microarray. Binding specificities of glycan-binding proteins (GBPs) including plant lectins and selected anti-glycan antibodies towards these O-GalNAc glycans are revealed by this microarray, promoting their applicability in functional O-glycomics. Serum samples from colorectal cancer patients and healthy controls are assayed using the array reveal higher bindings towards less common cores 3, 4, and 6 than abundant cores 1 and 2, providing insights into O-GalNAc glycan structure-activity relationships.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Divergent synthesis of amino acid-linked O-GalNAc glycan core structures.Nat Protoc. 2024 Sep 26. doi: 10.1038/s41596-024-01051-6. Online ahead of print. Nat Protoc. 2024. PMID: 39327537 Review.
-
Cellular O-Glycome Reporter/Amplification (CORA): Analytical and Preparative Tools to Study Mucin-Type O-Glycans of Living Cells.Curr Protoc. 2021 Jun;1(6):e142. doi: 10.1002/cpz1.142. Curr Protoc. 2021. PMID: 34101390
-
Molecular Recognition of GalNAc in Mucin-Type O-Glycosylation.Acc Chem Res. 2023 Mar 7;56(5):548-560. doi: 10.1021/acs.accounts.2c00723. Epub 2023 Feb 23. Acc Chem Res. 2023. PMID: 36815693 Free PMC article. Review.
-
Quantitative O-glycomics based on improvement of the one-pot method for nonreductive O-glycan release and simultaneous stable isotope labeling with 1-(d0/d5)phenyl-3-methyl-5-pyrazolone followed by mass spectrometric analysis.J Proteomics. 2017 Jan 6;150:18-30. doi: 10.1016/j.jprot.2016.08.012. Epub 2016 Aug 29. J Proteomics. 2017. PMID: 27585995
-
Structural and Quantitative Characterization of Mucin-Type O-Glycans and the Identification of O-Glycosylation Sites in Bovine Submaxillary Mucin.Biomolecules. 2020 Apr 20;10(4):636. doi: 10.3390/biom10040636. Biomolecules. 2020. PMID: 32326134 Free PMC article.
Cited by
-
Divergent synthesis of amino acid-linked O-GalNAc glycan core structures.Nat Protoc. 2024 Sep 26. doi: 10.1038/s41596-024-01051-6. Online ahead of print. Nat Protoc. 2024. PMID: 39327537 Review.
-
Genetic and functional diversity of β-N-acetylgalactosamine-targeting glycosidases expanded by deep-sea metagenome analysis.Nat Commun. 2024 May 10;15(1):3543. doi: 10.1038/s41467-024-47653-2. Nat Commun. 2024. PMID: 38730244 Free PMC article.
-
Highly stereoselective α-glycosylation with GalN3 donors enabled collective synthesis of mucin-related tumor associated carbohydrate antigens.Chem Sci. 2024 Apr 2;15(17):6552-6561. doi: 10.1039/d4sc01348d. eCollection 2024 May 1. Chem Sci. 2024. PMID: 38699257 Free PMC article.
-
Serum antibody screening using glycan arrays.Chem Soc Rev. 2024 Mar 4;53(5):2603-2642. doi: 10.1039/d3cs00693j. Chem Soc Rev. 2024. PMID: 38305761 Review.
-
Oxidative Release of O-Glycans under Neutral Conditions for Analysis of Glycoconjugates Having Base-Sensitive Substituents.Anal Chem. 2023 Jun 13;95(23):8825-8833. doi: 10.1021/acs.analchem.3c00127. Epub 2023 Jun 1. Anal Chem. 2023. PMID: 37259796 Free PMC article.
References
-
- Brockhausen I., Stanley P. O-GalNAc glycans. In Essentials of Glycobiology (ed^(eds rd, et al.) (2015).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

