Human umbilical cord mesenchymal stem cell transfusion in immune non-responders with AIDS: a multicenter randomized controlled trial
- PMID: 34103473
- PMCID: PMC8187429
- DOI: 10.1038/s41392-021-00607-2
Human umbilical cord mesenchymal stem cell transfusion in immune non-responders with AIDS: a multicenter randomized controlled trial
Abstract
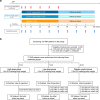
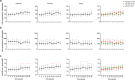
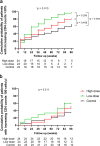
We examined the safety and efficacy of human umbilical cord mesenchymal stem cell (hUC-MSC) infusion for immune non-responder (INR) patients with chronic HIV-1 infection, who represent an unmet medical need even in the era of efficient antiretroviral therapy (ART). Seventy-two INR patients with HIV were enrolled in this phase II randomized, double-blinded, multicenter, placebo-controlled, dose-determination trial (NCT01213186) from May 2013 to March 2016. They were assigned to receive high-dose (1.5 × 106/kg body weight) or low-dose (0.5 × 106/kg body weight) hUC-MSC, or placebo. Their clinical and immunological parameters were monitored during the 96-week follow-up study. We found that hUC-MSC treatment was safe and well-tolerated. Compared with baseline, there was a statistical increase in CD4+ T counts in the high-dose (P < 0.001) and low-dose (P < 0.001) groups after 48-week treatment, but no change was observed in the control group. Kaplan-Meier analysis revealed a higher cumulative probability of achieving an immunological response in the low-dose group compared with the control group (95.8% vs. 70.8%, P = 0.004). However, no significant changes in CD4/CD8+ T counts and CD4/CD8 ratios were observed among the three groups. In summary, hUC-MSC treatment is safe. However, the therapeutic efficacy of hUC-MSC treatment to improve the immune reconstitution in INR patients still needs to be further investigated in a large cohort study.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Efficacy and safety of human umbilical cord-derived mesenchymal stem cells (hUC-MSC PLEB001) for the treatment of grade II-IV steroid-refractory acute graft-versus-host disease: a study protocol for a multicenter, randomized, double-blind, placebo-controlled, phase II trial.Trials. 2023 May 3;24(1):306. doi: 10.1186/s13063-023-07305-0. Trials. 2023. PMID: 37138332 Free PMC article.
-
Safety and efficacy of umbilical cord tissue-derived mesenchymal stem cells in the treatment of patients with aging frailty: a phase I/II randomized, double-blind, placebo-controlled study.Stem Cell Res Ther. 2024 Apr 29;15(1):122. doi: 10.1186/s13287-024-03707-2. Stem Cell Res Ther. 2024. PMID: 38679727 Free PMC article. Clinical Trial.
-
Extracellular Vesicles Released from Human Umbilical Cord-Derived Mesenchymal Stromal Cells Prevent Life-Threatening Acute Graft-Versus-Host Disease in a Mouse Model of Allogeneic Hematopoietic Stem Cell Transplantation.Stem Cells Dev. 2016 Dec 15;25(24):1874-1883. doi: 10.1089/scd.2016.0107. Epub 2016 Oct 24. Stem Cells Dev. 2016. PMID: 27649744
-
What is the impact of human umbilical cord mesenchymal stem cell transplantation on clinical treatment?Stem Cell Res Ther. 2020 Dec 1;11(1):519. doi: 10.1186/s13287-020-02011-z. Stem Cell Res Ther. 2020. PMID: 33261658 Free PMC article. Review.
-
Human umbilical cord mesenchymal stem cells: an overview of their potential in cell-based therapy.Expert Opin Biol Ther. 2015;15(9):1293-306. doi: 10.1517/14712598.2015.1051528. Epub 2015 Jun 12. Expert Opin Biol Ther. 2015. PMID: 26067213 Review.
Cited by
-
Promising Stem Cell therapy in the Management of HIV and AIDS: A Narrative Review.Biologics. 2022 Jul 8;16:89-105. doi: 10.2147/BTT.S368152. eCollection 2022. Biologics. 2022. PMID: 35836496 Free PMC article. Review.
-
Gaining momentum: stem cell therapies for HIV cure.Curr Opin HIV AIDS. 2024 Jul 1;19(4):194-200. doi: 10.1097/COH.0000000000000859. Epub 2024 Apr 26. Curr Opin HIV AIDS. 2024. PMID: 38686850 Free PMC article. Review.
-
Mesenchymal stem cells-based therapy in liver diseases.Mol Biomed. 2022 Jul 27;3(1):23. doi: 10.1186/s43556-022-00088-x. Mol Biomed. 2022. PMID: 35895169 Free PMC article. Review.
-
Incomplete immune reconstitution and its predictors in people living with HIV in Wuhan, China.BMC Public Health. 2023 Sep 16;23(1):1808. doi: 10.1186/s12889-023-16738-w. BMC Public Health. 2023. PMID: 37716975 Free PMC article.
-
Recent advances in poor HIV immune reconstitution: what will the future look like?Front Microbiol. 2023 Aug 7;14:1236460. doi: 10.3389/fmicb.2023.1236460. eCollection 2023. Front Microbiol. 2023. PMID: 37608956 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

