A comparative study of teriflunomide and dimethyl fumarate within the Swedish MS Registry
- PMID: 34080926
- PMCID: PMC8795225
- DOI: 10.1177/13524585211019649
A comparative study of teriflunomide and dimethyl fumarate within the Swedish MS Registry
Abstract
Background: Teriflunomide and dimethyl fumarate (DMF) are first-line disease-modifying treatments for multiple sclerosis with similar labels that are used in comparable populations.
Objectives: The objective of this study was to compare the effectiveness and persistence of teriflunomide and DMF in a Swedish real-world setting.
Methods: All relapsing-remitting multiple sclerosis (RRMS) patients in the Swedish MS registry initiating teriflunomide or DMF were included in the analysis. The primary endpoint was treatment persistence. Propensity score matching was used to adjust comparisons for baseline confounders.
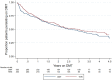
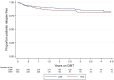
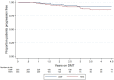
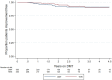
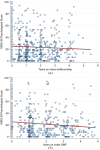
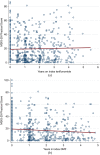
Results: A total of 353 teriflunomide patients were successfully matched to 353 DMF. There was no difference in the rate of overall treatment discontinuation by treatment group across the entire observation period (hazard ratio (HR) = 1.12; 95% confidence interval (CI) = 0.91-1.39; p = 0.277; reference = teriflunomide). Annualised relapse rate (ARR) was comparable (p = 0.237) between DMF (0.07; 95% CI = 0.05-0.10) and teriflunomide (0.09; 95% CI = 0.07-0.12). There was no difference in time to first on-treatment relapse (HR = 0.78; 95% CI = 0.50-1.21), disability progression (HR = 0.55; 95% CI = 0.27-1.12) or confirmed improvement (HR = 1.17; 95% CI = 0.57-2.36).
Conclusion: This population-based real-world study reports similarities in treatment persistence, clinical effectiveness and quality of life outcomes between teriflunomide and dimethyl fumarate.
Keywords: Dimethyl fumarate; multiple sclerosis; teriflunomide; treatment response.
Conflict of interest statement
Figures
Similar articles
-
Real world comparison of teriflunomide and dimethyl fumarate in naïve relapsing multiple sclerosis patients: Evidence from the Italian MS register.Mult Scler Relat Disord. 2022 Feb;58:103489. doi: 10.1016/j.msard.2022.103489. Epub 2022 Jan 2. Mult Scler Relat Disord. 2022. PMID: 35032879
-
Real-World Adherence and Persistence to Oral Disease-Modifying Therapies in Multiple Sclerosis Patients Over 1 Year.J Manag Care Spec Pharm. 2017 Aug;23(8):844-852. doi: 10.18553/jmcp.2017.23.8.844. J Manag Care Spec Pharm. 2017. PMID: 28737986 Free PMC article.
-
Real-life use of oral disease-modifying treatments in Austria.Acta Neurol Scand. 2019 Jul;140(1):32-39. doi: 10.1111/ane.13097. Epub 2019 Apr 22. Acta Neurol Scand. 2019. PMID: 30958901 Free PMC article.
-
Oral disease-modifying therapies for relapsing-remitting multiple sclerosis.Am J Health Syst Pharm. 2015 Jan 1;72(1):25-38. doi: 10.2146/ajhp140023. Am J Health Syst Pharm. 2015. PMID: 25511835 Review.
-
Teriflunomide for multiple sclerosis.Cochrane Database Syst Rev. 2012 Dec 12;12:CD009882. doi: 10.1002/14651858.CD009882.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2016 Mar 22;3:CD009882. doi: 10.1002/14651858.CD009882.pub3. PMID: 23235682 Updated. Review.
Cited by
-
Five-year efficacy outcomes of ocrelizumab in relapsing multiple sclerosis: A propensity-matched comparison of the OPERA studies with other disease-modifying therapies in real-world lines of treatments.J Cent Nerv Syst Dis. 2024 Sep 13;16:11795735241260563. doi: 10.1177/11795735241260563. eCollection 2024. J Cent Nerv Syst Dis. 2024. PMID: 39290861 Free PMC article.
-
Dimethyl Fumarate or Teriflunomide for Relapsing-Remitting Multiple Sclerosis: A Meta-analysis of Post-marketing Studies.Neurotherapeutics. 2023 Sep;20(5):1275-1283. doi: 10.1007/s13311-023-01416-x. Epub 2023 Aug 1. Neurotherapeutics. 2023. PMID: 37528262 Free PMC article.
-
Relationship between Patient Preferences, Attitudes to Treatment, Adherence, and Quality of Life in New Users of Teriflunomide.Pharmaceuticals (Basel). 2022 Oct 11;15(10):1248. doi: 10.3390/ph15101248. Pharmaceuticals (Basel). 2022. PMID: 36297360 Free PMC article.
-
PHREND®-A Real-World Data-Driven Tool Supporting Clinical Decisions to Optimize Treatment in Relapsing-Remitting Multiple Sclerosis.Front Digit Health. 2022 Mar 11;4:856829. doi: 10.3389/fdgth.2022.856829. eCollection 2022. Front Digit Health. 2022. PMID: 35360367 Free PMC article.
References
-
- Confavreux C, O’Connor P, Comi G, et al.. Oral teriflunomide for patients with relapsing multiple sclerosis (TOWER): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol 2014; 13(3): 247–256. - PubMed
-
- O’Connor P, Wolinsky JS, Confavreux C, et al.. Randomized trial of oral teriflunomide for relapsing multiple sclerosis. New Engl J Med 2011; 365(14): 1293–1303. - PubMed
-
- Vermersch P, Czlonkowska A, Grimaldi LM, et al.. Teriflunomide versus subcutaneous interferon beta-1a in patients with relapsing multiple sclerosis: A randomised, controlled phase 3 trial. Mult Scler 2014; 20(6): 705–716. - PubMed
-
- Miller AE, Wolinsky JS, Kappos L, et al.. Oral teriflunomide for patients with a first clinical episode suggestive of multiple sclerosis (TOPIC): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol 2014; 13(10): 977–986. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

