The Release of Peripheral Immune Inflammatory Cytokines Promote an Inflammatory Cascade in PCOS Patients via Altering the Follicular Microenvironment
- PMID: 34079559
- PMCID: PMC8165443
- DOI: 10.3389/fimmu.2021.685724
The Release of Peripheral Immune Inflammatory Cytokines Promote an Inflammatory Cascade in PCOS Patients via Altering the Follicular Microenvironment
Abstract
Background: Hormones and immune imbalance are critical factors in polycystic ovary syndrome (PCOS). The alternation of immune microenvironment of oocytes may play a significant role in infertility of PCOS patients.
Objective: This study explores the role of follicular fluid microenvironment change in inflammatory pathways activation of granulosa cells (GCs) in PCOS women infertility.
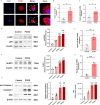
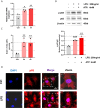
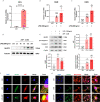
Methods: We enrolled 27 PCOS patients and 30 controls aged 22 to 38 years who underwent IVF and collected their luteinized granulosa cells (LGCs). Meanwhile, a granulosa-like tumor cell line (KGN) as a cell-model assisted this study. Key inflammatory markers in human ovarian GCs and follicular fluid were detected by RT-qPCR, Western blotting, or ELISA. The KGN cells were treated with follicle supernatant mixed with normal medium to simulate the microenvironment of GCs in PCOS patients, and the inflammation indicators were observed. The assembly of NLRP3 inflammasomes was detected by immunofluorescence techniques. Dihydroethidium assay and EdU proliferation assay were used to detect ROS and cell proliferation by flow cytometry.
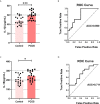
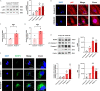
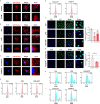
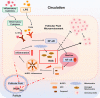
Results: Compared with normal controls (n = 19), IL-1β (P = 0.0005) and IL-18 (P = 0.021) in the follicular fluid of PCOS patients (n = 20) were significantly increased. The NF-κB pathway was activated, and NLRP3 inflammasome was formatted in ovarian GCs of PCOS patients. We also found that inflammation of KGN cells was activated with LPS irritation or stimulated by follicular fluid from PCOS patients. Finally, we found that intracellular inflammation process damaged mitochondrial structure and function, which induced oxidative stress, affected cellular metabolism, and impaired cell proliferation.
Conclusion: Inflammatory microenvironment alteration in the follicular fluid of PCOS patients leads to activated inflammatory pathway in GCs, serving as a crucial factor that causes adverse symptoms in patients. This study provides a novel mechanism in the inflammatory process of PCOS.
Keywords: NF-κB; NLRP3 inflammasomes; follicular fluid (FF); granulosa cells (GCs); microenvironment; polycystic ovary syndrome (PCOS).
Copyright © 2021 Liu, Liu, Li, Fan, Yan, Liu, Xuan, Feng and Wei.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Chitosan oligosaccharide improves ovarian granulosa cells inflammation and oxidative stress in patients with polycystic ovary syndrome.Front Immunol. 2023 Mar 1;14:1086232. doi: 10.3389/fimmu.2023.1086232. eCollection 2023. Front Immunol. 2023. PMID: 36936973 Free PMC article.
-
Elevated Levels of Follicular Fatty Acids Induce Ovarian Inflammation via ERK1/2 and Inflammasome Activation in PCOS.J Clin Endocrinol Metab. 2022 Jul 14;107(8):2307-2317. doi: 10.1210/clinem/dgac281. J Clin Endocrinol Metab. 2022. PMID: 35521772
-
Dopamine in human follicular fluid is associated with cellular uptake and metabolism-dependent generation of reactive oxygen species in granulosa cells: implications for physiology and pathology.Hum Reprod. 2014 Mar;29(3):555-67. doi: 10.1093/humrep/det422. Epub 2013 Nov 27. Hum Reprod. 2014. PMID: 24287819
-
Disturbed Follicular Microenvironment in Polycystic Ovary Syndrome: Relationship to Oocyte Quality and Infertility.Endocrinology. 2024 Feb 20;165(4):bqae023. doi: 10.1210/endocr/bqae023. Endocrinology. 2024. PMID: 38375912 Review.
-
Inducers and Inhibitors of Pyroptotic Death of Granulosa Cells in Models of Premature Ovarian Insufficiency and Polycystic Ovary Syndrome.Reprod Sci. 2024 Oct;31(10):2972-2992. doi: 10.1007/s43032-024-01643-3. Epub 2024 Jul 18. Reprod Sci. 2024. PMID: 39026050 Free PMC article. Review.
Cited by
-
The interplay between androgens and the immune response in polycystic ovary syndrome.J Transl Med. 2023 Apr 16;21(1):259. doi: 10.1186/s12967-023-04116-4. J Transl Med. 2023. PMID: 37062827 Free PMC article. Review.
-
Transcriptomic responses of cumulus granulosa cells to SARS-CoV-2 infection during controlled ovarian stimulation.Apoptosis. 2024 Jun;29(5-6):649-662. doi: 10.1007/s10495-024-01942-9. Epub 2024 Feb 26. Apoptosis. 2024. PMID: 38409352
-
Short-Term Metformin Therapy in Clomiphene Citrate Resistant PCOS Patients Improves Fertility Outcome by Regulating Follicular Fluid Redox Balance: A Case-Controlled Study.Diagnostics (Basel). 2024 Oct 4;14(19):2215. doi: 10.3390/diagnostics14192215. Diagnostics (Basel). 2024. PMID: 39410619 Free PMC article.
-
Effects of interleukin-1 receptor antagonism in women with polycystic ovary syndrome-the FertIL trial.Front Endocrinol (Lausanne). 2024 Sep 11;15:1435698. doi: 10.3389/fendo.2024.1435698. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39324125 Free PMC article. Clinical Trial.
-
Advancements in lead therapeutic phytochemicals polycystic ovary syndrome: A review.Front Pharmacol. 2023 Jan 9;13:1065243. doi: 10.3389/fphar.2022.1065243. eCollection 2022. Front Pharmacol. 2023. PMID: 36699064 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

