Elevating the Levels of Calcium Ions Exacerbate Alzheimer's Disease via Inducing the Production and Aggregation of β-Amyloid Protein and Phosphorylated Tau
- PMID: 34072743
- PMCID: PMC8198078
- DOI: 10.3390/ijms22115900
Elevating the Levels of Calcium Ions Exacerbate Alzheimer's Disease via Inducing the Production and Aggregation of β-Amyloid Protein and Phosphorylated Tau
Abstract
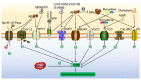
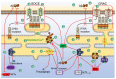
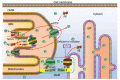
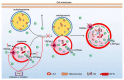
Alzheimer's disease (AD) is a neurodegenerative disease with a high incidence rate. The main pathological features of AD are β-amyloid plaques (APs), which are formed by β-amyloid protein (Aβ) deposition, and neurofibrillary tangles (NFTs), which are formed by the excessive phosphorylation of the tau protein. Although a series of studies have shown that the accumulation of metal ions, including calcium ions (Ca2+), can promote the formation of APs and NFTs, there is no systematic review of the mechanisms by which Ca2+ affects the development and progression of AD. In view of this, the current review summarizes the mechanisms by which Ca2+ is transported into and out of cells and organelles, such as the cell, endoplasmic reticulum, mitochondrial and lysosomal membranes to affect the balance of intracellular Ca2+ levels. In addition, dyshomeostasis of Ca2+ plays an important role in modulating the pathogenesis of AD by influencing the production and aggregation of Aβ peptides and tau protein phosphorylation and the ways that disrupting the metabolic balance of Ca2+ can affect the learning ability and memory of people with AD. In addition, the effects of these mechanisms on the synaptic plasticity are also discussed. Finally, the molecular network through which Ca2+ regulates the pathogenesis of AD is introduced, providing a theoretical basis for improving the clinical treatment of AD.
Keywords: Alzheimer’s disease; calcium ions; mechanisms; review; transporters.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Similar articles
-
Calcium signaling in Alzheimer's disease & therapies.Biochim Biophys Acta Mol Cell Res. 2018 Nov;1865(11 Pt B):1745-1760. doi: 10.1016/j.bbamcr.2018.07.018. Epub 2018 Jul 29. Biochim Biophys Acta Mol Cell Res. 2018. PMID: 30059692 Review.
-
Amyloid Beta and Phosphorylated Tau-Induced Defective Autophagy and Mitophagy in Alzheimer's Disease.Cells. 2019 May 22;8(5):488. doi: 10.3390/cells8050488. Cells. 2019. PMID: 31121890 Free PMC article. Review.
-
Metal ions influx is a double edged sword for the pathogenesis of Alzheimer's disease.Ageing Res Rev. 2017 May;35:265-290. doi: 10.1016/j.arr.2016.10.003. Epub 2016 Nov 6. Ageing Res Rev. 2017. PMID: 27829171 Review.
-
Review on Alzheimer's disease: Inhibition of amyloid beta and tau tangle formation.Int J Biol Macromol. 2021 Jan 15;167:382-394. doi: 10.1016/j.ijbiomac.2020.11.192. Epub 2020 Dec 2. Int J Biol Macromol. 2021. PMID: 33278431 Review.
-
Synaptic Mitochondria: An Early Target of Amyloid-β and Tau in Alzheimer's Disease.J Alzheimers Dis. 2021;84(4):1391-1414. doi: 10.3233/JAD-215139. J Alzheimers Dis. 2021. PMID: 34719499 Review.
Cited by
-
Trends and hotspots in tea and Alzheimer's disease research from 2014 to 2023: A bibliometric and visual analysis.Heliyon. 2024 Apr 23;10(9):e30063. doi: 10.1016/j.heliyon.2024.e30063. eCollection 2024 May 15. Heliyon. 2024. PMID: 38699003 Free PMC article. Review.
-
Autophagy-lysosomal pathway impairment and cathepsin dysregulation in Alzheimer's disease.Front Mol Biosci. 2024 Oct 31;11:1490275. doi: 10.3389/fmolb.2024.1490275. eCollection 2024. Front Mol Biosci. 2024. PMID: 39544403 Free PMC article. Review.
-
Potential mechanisms underlying lithium treatment for Alzheimer's disease and COVID-19.Eur Rev Med Pharmacol Sci. 2022 Mar;26(6):2201-2214. doi: 10.26355/eurrev_202203_28369. Eur Rev Med Pharmacol Sci. 2022. PMID: 35363371 Free PMC article. Review.
-
Alzheimer's Disease beyond Calcium Dysregulation: The Complex Interplay between Calmodulin, Calmodulin-Binding Proteins and Amyloid Beta from Disease Onset through Progression.Curr Issues Mol Biol. 2023 Jul 27;45(8):6246-6261. doi: 10.3390/cimb45080393. Curr Issues Mol Biol. 2023. PMID: 37623212 Free PMC article. Review.
-
Polymeric nanoparticles: A promising strategy for treatment of Alzheimer's disease.J Taibah Univ Med Sci. 2024 Apr 26;19(3):549-565. doi: 10.1016/j.jtumed.2024.04.004. eCollection 2024 Jun. J Taibah Univ Med Sci. 2024. PMID: 38736898 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

