Translational and pharmacokinetic-pharmacodynamic application for the clinical development of GDC-0334, a novel TRPA1 inhibitor
- PMID: 34058071
- PMCID: PMC8504827
- DOI: 10.1111/cts.13049
Translational and pharmacokinetic-pharmacodynamic application for the clinical development of GDC-0334, a novel TRPA1 inhibitor
Abstract
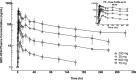
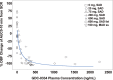
GDC-0334 is a novel small molecule inhibitor of transient receptor potential cation channel member A1 (TRPA1), a promising therapeutic target for many nervous system and respiratory diseases. The pharmacokinetic (PK) profile and pharmacodynamic (PD) effects of GDC-0334 were evaluated in this first-in-human (FIH) study. A starting single dose of 25 mg was selected based on integrated preclinical PK, PD, and toxicology data following oral administration of GDC-0334 in guinea pigs, rats, dogs, and monkeys. Human PK and PK-PD of GDC-0334 were characterized after single and multiple oral dosing using a population modeling approach. The ability of GDC-0334 to inhibit dermal blood flow (DBF) induced by topical administration of allyl isothiocyanate (AITC) was evaluated as a target-engagement biomarker. Quantitative models were developed iteratively to refine the parameter estimates of the dose-concentration-effect relationships through stepwise estimation and extrapolation. Human PK analyses revealed that bioavailability, absorption rate constant, and lag time increase when GDC-0334 was administered with food. The inhibitory effect of GDC-0334 on the AITC-induced DBF biomarker exhibited a clear sigmoid-Emax relationship with GDC-0334 plasma concentrations in humans. This study leveraged emerging preclinical and clinical data to enable iterative refinement of GDC-0334 mathematical models throughout the FIH study for dose selection in subsequent cohorts throughout the study. Study Highlights WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC? GDC-0334 is a novel, small molecule TRPA1 inhibitor and a pharmacokinetic-pharmacodynamic (PK-PD) modeling strategy could be implemented in a systematic and step-wise manner to build and learn from emerging data for early clinical development. WHAT QUESTION DID THIS STUDY ADDRESS? Can noncompartmental and population-based analyses be used to describe the PK and PD characteristics of GDC-0334 in preclinical and clinical studies? WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE? GDC-0334 exposure generally increased with dose in rats, dogs, and monkeys. The starting dose (25 mg) in the clinical study was determined based on the preclinical data. GDC-0334 exhibited linear PK in humans and the bioavailability was increased with food. The inhibitory effect of GDC-0334 on dermal blood flow induced by the TRPA1 agonist allyl isothiocyanate in humans indicates a clear PK-PD relationship. HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE? The models developed based on TRPA1 agonist-induced dermal blood flow inhibition data can be used to predict PK-PD relationships in future preclinical and clinical studies evaluating new drug entities that target TRPA1.
© 2021 Genentech. Clinical and Translational Science published by Wiley Periodicals LLC on behalf of the American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
All authors are current or former employees and stockholders of Genentech/Roche.
Figures

Similar articles
-
A TRPA1 inhibitor suppresses neurogenic inflammation and airway contraction for asthma treatment.J Exp Med. 2021 Apr 5;218(4):e20201637. doi: 10.1084/jem.20201637. J Exp Med. 2021. PMID: 33620419 Free PMC article. Clinical Trial.
-
Development of AITC-induced dermal blood flow as a translational in vivo biomarker of TRPA1 activity in human and rodent skin.Br J Clin Pharmacol. 2021 Jan;87(1):129-139. doi: 10.1111/bcp.14370. Epub 2020 Jun 2. Br J Clin Pharmacol. 2021. PMID: 32415670
-
Learning and confirming with preclinical studies: modeling and simulation in the discovery of GDC-0917, an inhibitor of apoptosis proteins antagonist.Drug Metab Dispos. 2013 Dec;41(12):2104-13. doi: 10.1124/dmd.113.053926. Epub 2013 Sep 16. Drug Metab Dispos. 2013. PMID: 24041744 Clinical Trial.
-
Mechanistic prediction of first-in-human dose for bispecific CD3/EpCAM T-cell engager antibody M701, using an integrated PK/PD modeling method.Eur J Pharm Sci. 2021 Mar 1;158:105584. doi: 10.1016/j.ejps.2020.105584. Epub 2020 Oct 9. Eur J Pharm Sci. 2021. PMID: 33039565 Review.
-
Basic concepts of pharmacokinetic/pharmacodynamic (PK/PD) modelling.Int J Clin Pharmacol Ther. 1997 Oct;35(10):401-13. Int J Clin Pharmacol Ther. 1997. PMID: 9352388 Review.
Cited by
-
Human Transient Receptor Potential Ankyrin 1 Channel: Structure, Function, and Physiology.Subcell Biochem. 2024;104:207-244. doi: 10.1007/978-3-031-58843-3_10. Subcell Biochem. 2024. PMID: 38963489 Review.
-
Open-Label Interventional Study in Healthy Volunteers to Evaluate NO-Mediated Vasodilation by Dermal Allyl Isothiocyanate Challenge and Whole-Body Heat Stress.J Exp Pharmacol. 2024 Sep 17;16:285-294. doi: 10.2147/JEP.S473217. eCollection 2024. J Exp Pharmacol. 2024. PMID: 39308849 Free PMC article. Clinical Trial.
-
Neuronal and non-neuronal TRPA1 as therapeutic targets for pain and headache relief.Temperature (Austin). 2022 May 29;10(1):50-66. doi: 10.1080/23328940.2022.2075218. eCollection 2023. Temperature (Austin). 2022. PMID: 37187829 Free PMC article. Review.
-
Discovery of BAY-390, a Selective CNS Penetrant Chemical Probe as Transient Receptor Potential Ankyrin 1 (TRPA1) Antagonist.J Med Chem. 2023 Jan 26;66(2):1583-1600. doi: 10.1021/acs.jmedchem.2c01830. Epub 2023 Jan 9. J Med Chem. 2023. PMID: 36622903 Free PMC article.
-
TRP (transient receptor potential) ion channel family: structures, biological functions and therapeutic interventions for diseases.Signal Transduct Target Ther. 2023 Jul 5;8(1):261. doi: 10.1038/s41392-023-01464-x. Signal Transduct Target Ther. 2023. PMID: 37402746 Free PMC article. Review.
References
-
- Wong H, Bohnert T, Damian‐Iordache V, et al. Translational pharmacokinetic‐pharmacodynamic analysis in the pharmaceutical industry: an IQ Consortium PK‐PD Discussion Group perspective. Drug Dis Today. 2017;22:1447‐1459. - PubMed
-
- Moran MM, McAlexander MA, Bíró T, Szallasi A. Transient receptor potential channels as therapeutic targets. Nat Rev Drug Disc. 2011;10:601‐620. - PubMed
-
- Verma VA, Shore DGM, Chen H et al. α‐Aryl pyrrolidine sulfonamides as TRPA1 antagonists. Bioorganic Med Chem Lett. 2016;26:495‐498. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

