Posaconazole for prevention of invasive pulmonary aspergillosis in critically ill influenza patients (POSA-FLU): a randomised, open-label, proof-of-concept trial
- PMID: 34050768
- PMCID: PMC8164057
- DOI: 10.1007/s00134-021-06431-0
Posaconazole for prevention of invasive pulmonary aspergillosis in critically ill influenza patients (POSA-FLU): a randomised, open-label, proof-of-concept trial
Abstract
Purpose: Influenza-associated pulmonary aspergillosis (IAPA) is a frequent complication in critically ill influenza patients, associated with significant mortality. We investigated whether antifungal prophylaxis reduces the incidence of IAPA.
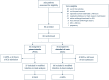
Methods: We compared 7 days of intravenous posaconazole (POS) prophylaxis with no prophylaxis (standard-of-care only, SOC) in a randomised, open-label, proof-of-concept trial in patients admitted to an intensive care unit (ICU) with respiratory failure due to influenza (ClinicalTrials.gov, NCT03378479). Adult patients with PCR-confirmed influenza were block randomised (1:1) within 10 days of symptoms onset and 48 h of ICU admission. The primary endpoint was the incidence of IAPA during ICU stay in patients who did not have IAPA within 48 h of ICU admission (modified intention-to-treat (MITT) population).
Results: Eighty-eight critically ill influenza patients were randomly allocated to POS or SOC. IAPA occurred in 21 cases (24%), the majority of which (71%, 15/21) were diagnosed within 48 h of ICU admission, excluding them from the MITT population. The incidence of IAPA was not significantly reduced in the POS arm (5.4%, 2/37) compared with SOC (11.1%, 4/36; between-group difference 5.7%; 95% CI - 10.8 to 21.7; p = 0.32). ICU mortality of early IAPA was high (53%), despite rapid antifungal treatment.
Conclusion: The higher than expected incidence of early IAPA precludes any definite conclusion on POS prophylaxis. High mortality of early IAPA, despite timely antifungal therapy, indicates that alternative management strategies are required. After 48 h, still 11% of patients developed IAPA. As these could benefit from prophylaxis, differentiated strategies are likely needed to manage IAPA in the ICU.
Keywords: Aspergillosis; Critical illness; Influenza; Posaconazole; Prophylaxis.
Conflict of interest statement
JBB reports grants from F2G, grants from Gilead Sciences, grants from Thermo Fisher Scientific, outside the submitted work. KL received consultancy fees from MSD, SMB Laboratoires Brussels and Gilead, non-financial support from Pfizer and MSD, received speaker fees from Gilead Sciences, FUJIFILM WAKO and Pfizer and a grant from Thermo Fisher Scientific. VL reports financial support of Pfizer, Fisher Paykel, Gilead Sciences, Alexion and Celgene to her research group, outside the submitted work. JM reports grants, personal fees and non-financial support from Gilead Sciences, MSD and Pfizer; personal fees and non-financial support from Cidara, F2G and Mundipharma; outside the submitted work. AWT reports personal fees and non-financial support from Fisher&Paykel, outside the submitted work. RVD reports non-financial support from Pfizer and Gilead Sciences, outside the submitted work. LV reports non-financial support from Gilead Sciences, outside the submitted work. PEV reports grants from Mundipharma, grants from F2G, grants from Pfizer, grants from Thermofisher, grants from Gilead Sciences, non-financial support from IMMY, grants from Cidara, outside the submitted work. RJMB reports consultancy fees from Mundipharma, Cidara, Amplyx, F2G, Gilead, Pfizer and MSD, speaker fees from Pfizer and Gilead, grants from Pfizer, Gilead and MSD. JW received speakers fee from MSD, Pfizer and Gilead, consultancy fee from Gilead and he obtained investigator-initiated grants from Gilead, Pfizer and MSD.
Figures

Comment in
-
Influenza and prophylactic antifungal therapy for aspergillosis: addressing some questions first.Intensive Care Med. 2021 Nov;47(11):1341-1342. doi: 10.1007/s00134-021-06488-x. Epub 2021 Aug 10. Intensive Care Med. 2021. PMID: 34374834 No abstract available.
-
Critical influenza and prophylactic antifungal therapy for aspergillosis: a nuanced approach to a pertinent infectious disease.Intensive Care Med. 2021 Nov;47(11):1343-1344. doi: 10.1007/s00134-021-06532-w. Epub 2021 Oct 4. Intensive Care Med. 2021. PMID: 34608528 No abstract available.
Similar articles
-
Higher risk for influenza-associated pulmonary aspergillosis (IAPA) in asthmatic patients: A Swiss multicenter cohort study on IAPA in critically ill influenza patients.Influenza Other Respir Viruses. 2023 Jan;17(1):e13059. doi: 10.1111/irv.13059. Epub 2022 Nov 16. Influenza Other Respir Viruses. 2023. PMID: 36394086 Free PMC article.
-
Clinical characteristics and outcomes of influenza-associated pulmonary aspergillosis among critically ill patients: a systematic review and meta-analysis.J Hosp Infect. 2022 Feb;120:98-109. doi: 10.1016/j.jhin.2021.11.016. Epub 2021 Nov 26. J Hosp Infect. 2022. PMID: 34843812 Review.
-
Prevalence, Risk Factors, Clinical Features, and Outcome of Influenza-Associated Pulmonary Aspergillosis in Critically Ill Patients: A Systematic Review and Meta-Analysis.Chest. 2024 Mar;165(3):540-558. doi: 10.1016/j.chest.2023.09.019. Epub 2023 Sep 22. Chest. 2024. PMID: 37742914
-
Influenza-associated invasive aspergillosis in patients admitted to the intensive care unit in Sweden: a prospective multicentre cohort study.Infect Dis (Lond). 2024 Feb;56(2):110-115. doi: 10.1080/23744235.2023.2273381. Epub 2023 Dec 18. Infect Dis (Lond). 2024. PMID: 37897800
-
Review of influenza-associated pulmonary aspergillosis in ICU patients and proposal for a case definition: an expert opinion.Intensive Care Med. 2020 Aug;46(8):1524-1535. doi: 10.1007/s00134-020-06091-6. Epub 2020 Jun 22. Intensive Care Med. 2020. PMID: 32572532 Free PMC article.
Cited by
-
Higher risk for influenza-associated pulmonary aspergillosis (IAPA) in asthmatic patients: A Swiss multicenter cohort study on IAPA in critically ill influenza patients.Influenza Other Respir Viruses. 2023 Jan;17(1):e13059. doi: 10.1111/irv.13059. Epub 2022 Nov 16. Influenza Other Respir Viruses. 2023. PMID: 36394086 Free PMC article.
-
Personalized Antifungal Therapy Through Model-Informed Precision Dosing of Posaconazole.Clin Pharmacokinet. 2024 May;63(5):645-656. doi: 10.1007/s40262-024-01361-8. Epub 2024 Mar 26. Clin Pharmacokinet. 2024. PMID: 38532053 Free PMC article.
-
Dysregulated Pulmonary Inflammatory Responses Exacerbate the Outcome of Secondary Aspergillosis Following Influenza.bioRxiv [Preprint]. 2023 Jun 30:2023.06.27.546808. doi: 10.1101/2023.06.27.546808. bioRxiv. 2023. Update in: mBio. 2023 Oct 31;14(5):e0163323. doi: 10.1128/mbio.01633-23. PMID: 37425745 Free PMC article. Updated. Preprint.
-
COVID-19 Syndemic: Convergence of COVID-19, Pulmonary Aspergillosis (CAPA), Pulmonary Tuberculosis, Type 2 Diabetes Mellitus, and Arterial Hypertension.Diagnostics (Basel). 2022 Aug 25;12(9):2058. doi: 10.3390/diagnostics12092058. Diagnostics (Basel). 2022. PMID: 36140460 Free PMC article.
-
Diagnosis and Antifungal Prophylaxis for COVID-19 Associated Pulmonary Aspergillosis.Antibiotics (Basel). 2022 Nov 26;11(12):1704. doi: 10.3390/antibiotics11121704. Antibiotics (Basel). 2022. PMID: 36551361 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

