Aged hematopoietic stem cells are refractory to bloodborne systemic rejuvenation interventions
- PMID: 34032859
- PMCID: PMC8155813
- DOI: 10.1084/jem.20210223
Aged hematopoietic stem cells are refractory to bloodborne systemic rejuvenation interventions
Abstract
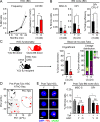
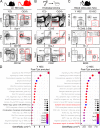
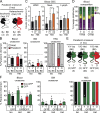
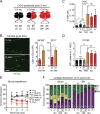
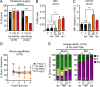
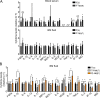
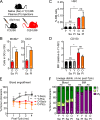
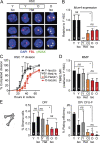
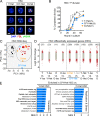
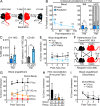
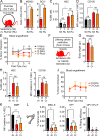
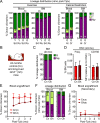
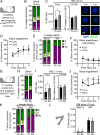
While young blood can restore many aged tissues, its effects on the aged blood system itself and old hematopoietic stem cells (HSCs) have not been determined. Here, we used transplantation, parabiosis, plasma transfer, exercise, calorie restriction, and aging mutant mice to understand the effects of age-regulated systemic factors on HSCs and their bone marrow (BM) niche. We found that neither exposure to young blood, nor long-term residence in young niches after parabiont separation, nor direct heterochronic transplantation had any observable rejuvenating effects on old HSCs. Likewise, exercise and calorie restriction did not improve old HSC function, nor old BM niches. Conversely, young HSCs were not affected by systemic pro-aging conditions, and HSC function was not impacted by mutations influencing organismal aging in established long-lived or progeroid genetic models. Therefore, the blood system that carries factors with either rejuvenating or pro-aging properties for many other tissues is itself refractory to those factors.
© 2021 Ho et al.
Conflict of interest statement
Disclosures: E.V. Verovskaya is currently an employee of Thermo Fisher Scientific. No other disclosures were reported.
Figures


Similar articles
-
Limited rejuvenation of aged hematopoietic stem cells in young bone marrow niche.J Exp Med. 2021 Mar 1;218(3):e20192283. doi: 10.1084/jem.20192283. J Exp Med. 2021. PMID: 33231616 Free PMC article.
-
NOD Scid Gamma Mice Are Permissive to Allogeneic HSC Transplantation without Prior Conditioning.Int J Mol Sci. 2016 Nov 7;17(11):1850. doi: 10.3390/ijms17111850. Int J Mol Sci. 2016. PMID: 27827995 Free PMC article.
-
Mechanisms and rejuvenation strategies for aged hematopoietic stem cells.J Hematol Oncol. 2020 Apr 6;13(1):31. doi: 10.1186/s13045-020-00864-8. J Hematol Oncol. 2020. PMID: 32252797 Free PMC article. Review.
-
Heterochronic parabiosis induces stem cell revitalization and systemic rejuvenation across aged tissues.Cell Stem Cell. 2022 Jun 2;29(6):990-1005.e10. doi: 10.1016/j.stem.2022.04.017. Epub 2022 May 24. Cell Stem Cell. 2022. PMID: 35613617
-
Aging and brain rejuvenation as systemic events.J Neurochem. 2015 Jan;132(1):5-19. doi: 10.1111/jnc.12969. Epub 2014 Dec 5. J Neurochem. 2015. PMID: 25327899 Free PMC article. Review.
Cited by
-
Epidermal growth factor augments the self-renewal capacity of aged hematopoietic stem cells.iScience. 2024 Jun 19;27(7):110306. doi: 10.1016/j.isci.2024.110306. eCollection 2024 Jul 19. iScience. 2024. PMID: 39055915 Free PMC article.
-
Blood-to-brain communication in aging and rejuvenation.Nat Neurosci. 2023 Mar;26(3):379-393. doi: 10.1038/s41593-022-01238-8. Epub 2023 Jan 16. Nat Neurosci. 2023. PMID: 36646876 Review.
-
Induction of mitochondrial recycling reverts age-associated decline of the hematopoietic and immune systems.Nat Aging. 2023 Sep;3(9):1057-1066. doi: 10.1038/s43587-023-00473-3. Epub 2023 Aug 31. Nat Aging. 2023. PMID: 37653255
-
Aging brain: exploring the interplay between bone marrow aging, immunosenescence, and neuroinflammation.Front Immunol. 2024 Apr 4;15:1393324. doi: 10.3389/fimmu.2024.1393324. eCollection 2024. Front Immunol. 2024. PMID: 38638424 Free PMC article. Review.
-
Taz protects hematopoietic stem cells from an aging-dependent decrease in PU.1 activity.Nat Commun. 2022 Sep 3;13(1):5187. doi: 10.1038/s41467-022-32970-1. Nat Commun. 2022. PMID: 36057685 Free PMC article.
References
-
- Baker, D.J., Jeganathan K.B., Cameron J.D., Thompson M., Juneja S., Kopecka A., Kumar R., Jenkins R.B., de Groen P.C., Roche P., and van Deursen J.M.. 2004. BubR1 insufficiency causes early onset of aging-associated phenotypes and infertility in mice. Nat. Genet. 36:744–749. 10.1038/ng1382 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

