Feasibility of short imaging protocols for [18F]PI-2620 tau-PET in progressive supranuclear palsy
- PMID: 34021393
- PMCID: PMC8484138
- DOI: 10.1007/s00259-021-05391-3
Feasibility of short imaging protocols for [18F]PI-2620 tau-PET in progressive supranuclear palsy
Abstract
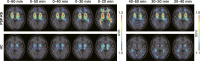
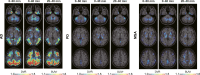
Purpose: Dynamic 60-min positron emission tomography (PET) imaging with the novel tau radiotracer [18F]PI-2620 facilitated accurate discrimination between patients with progressive supranuclear palsy (PSP) and healthy controls (HCs). This study investigated if truncated acquisition and static time windows can be used for [18F]PI-2620 tau-PET imaging of PSP.
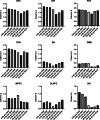
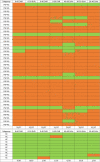
Methods: Thirty-seven patients with PSP Richardson syndrome (PSP-RS) were evaluated together with ten HCs. [18F]PI-2620 PET was performed by a dynamic 60-min scan. Distribution volume ratios (DVRs) were calculated using full and truncated scan durations (0-60, 0-50, 0-40, 0-30, and 0-20 min p.i.). Standardized uptake value ratios (SUVrs) were obtained 20-40, 30-50, and 40-60 min p.i.. All DVR and SUVr data were compared with regard to their potential to discriminate patients with PSP-RS from HCs in predefined subcortical and cortical target regions (effect size, area under the curve (AUC), multi-region classifier).
Results: 0-50 and 0-40 DVR showed equivalent effect sizes as 0-60 DVR (averaged Cohen's d: 1.22 and 1.16 vs. 1.26), whereas the performance dropped for 0-30 or 0-20 DVR. The 20-40 SUVr indicated the best performance of all static acquisition windows (averaged Cohen's d: 0.99). The globus pallidus internus discriminated patients with PSP-RS and HCs at a similarly high level for 0-60 DVR (AUC: 0.96), 0-40 DVR (AUC: 0.96), and 20-40 SUVr (AUC: 0.94). The multi-region classifier sensitivity of these time windows was consistently 86%.
Conclusion: Truncated and static imaging windows can be used for [18F]PI-2620 PET imaging of PSP. 0-40 min dynamic scanning offers the best balance between accuracy and economic scanning.
Keywords: Progressive supranuclear palsy; Tau-PET; Time window; [18F]PI-2620.
© 2021. The Author(s).
Conflict of interest statement
M.B. received speaker honoraria from GE healthcare and LMI and is an advisor of LMI. M.T.B. received speaker’s honoraria from Medtronic, Boston Scientific, Abbott (formerly St. Jude), GE Medical, UCB, Bial and research funding from the Felgenhauer-Stiftung, Forschungspool Klinische Studien (University of Cologne), H2020, Medtronic and Boston Scientific. C.P. received research funding from Lüneburg heritage. G.U.H. received research support from GE Healthcare and Neuropore; has ongoing research collaborations with Orion and Prothena; serves as a consultant for AbbVie, AlzProtect, Asceneuron, Biogen, Biohaven, Lundbeck, Novartis, Roche, Sanofi, and UCB; received honoraria for scientific presentations from AbbVie, Biogen, Roche, Teva, UCB, and Zambon; and holds a patent on PERK Activation for the Treatment of Neurodegenerative Diseases (PCT/EP2015/068734). G.R. received honoraria for scientific presentations from Biogen. O.S. receives research support from LMI. All other authors do not report a conflict of interest.
Figures

Similar articles
-
[18F]PI-2620 Binding Patterns in Patients with Suspected Alzheimer Disease and Frontotemporal Lobar Degeneration.J Nucl Med. 2023 Dec 1;64(12):1980-1989. doi: 10.2967/jnumed.123.265856. J Nucl Med. 2023. PMID: 37918868 Free PMC article.
-
Tau PET imaging in progressive supranuclear palsy: a systematic review and meta-analysis.J Neurol. 2023 May;270(5):2451-2467. doi: 10.1007/s00415-022-11556-3. Epub 2023 Jan 12. J Neurol. 2023. PMID: 36633672 Review.
-
18F-PI-2620 Tau PET Improves the Imaging Diagnosis of Progressive Supranuclear Palsy.J Nucl Med. 2022 Nov;63(11):1754-1760. doi: 10.2967/jnumed.121.262854. Epub 2022 Apr 14. J Nucl Med. 2022. PMID: 35422444 Free PMC article.
-
Assessment of 18F-PI-2620 as a Biomarker in Progressive Supranuclear Palsy.JAMA Neurol. 2020 Nov 1;77(11):1408-1419. doi: 10.1001/jamaneurol.2020.2526. JAMA Neurol. 2020. PMID: 33165511 Free PMC article.
-
New Perspectives in Radiological and Radiopharmaceutical Hybrid Imaging in Progressive Supranuclear Palsy: A Systematic Review.Cells. 2023 Dec 6;12(24):2776. doi: 10.3390/cells12242776. Cells. 2023. PMID: 38132096 Free PMC article. Review.
Cited by
-
Molecular Imaging in Parkinsonian Disorders-What's New and Hot?Brain Sci. 2022 Aug 27;12(9):1146. doi: 10.3390/brainsci12091146. Brain Sci. 2022. PMID: 36138882 Free PMC article. Review.
-
[18F]PI-2620 Binding Patterns in Patients with Suspected Alzheimer Disease and Frontotemporal Lobar Degeneration.J Nucl Med. 2023 Dec 1;64(12):1980-1989. doi: 10.2967/jnumed.123.265856. J Nucl Med. 2023. PMID: 37918868 Free PMC article.
-
Symptomatology in 4-repeat tauopathies is associated with data-driven topology of [18F]-PI-2620 tau-PET signal.Neuroimage Clin. 2023;38:103402. doi: 10.1016/j.nicl.2023.103402. Epub 2023 Apr 11. Neuroimage Clin. 2023. PMID: 37087820 Free PMC article.
-
Tau in Atypical Parkinsonisms: A Meta-Analysis of in Vivo PET Imaging Findings.Mov Disord Clin Pract. 2023 Sep 29;10(12):1725-1737. doi: 10.1002/mdc3.13885. eCollection 2023 Dec. Mov Disord Clin Pract. 2023. PMID: 38094644 Free PMC article. Review.
-
Tau PET imaging in progressive supranuclear palsy: a systematic review and meta-analysis.J Neurol. 2023 May;270(5):2451-2467. doi: 10.1007/s00415-022-11556-3. Epub 2023 Jan 12. J Neurol. 2023. PMID: 36633672 Review.
References
-
- Steele JC, Richardson JC, Olszewski J. Progressive Supranuclear Palsy. A heterogeneous degeneration involving the brain stem, basal ganglia and cerebellum with vertical gaze and pseudobulbar palsy, Nuchal Dystonia and Dementia. Arch Neurol. 1964;10:333–359. doi: 10.1001/archneur.1964.00460160003001. - DOI - PubMed
-
- Kroth H, Oden F, Molette J, Schieferstein H, Capotosti F, Mueller A, et al. Discovery and preclinical characterization of [(18) F]PI-2620, a next-generation tau PET tracer for the assessment of tau pathology in Alzheimer's disease and other tauopathies. Eur J Nucl Med Mol Imaging. 2019;46:2178–2189. doi: 10.1007/s00259-019-04397-2. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

