SARS-CoV-2 Induces Lymphocytopenia by Promoting Inflammation and Decimates Secondary Lymphoid Organs
- PMID: 33995382
- PMCID: PMC8113960
- DOI: 10.3389/fimmu.2021.661052
SARS-CoV-2 Induces Lymphocytopenia by Promoting Inflammation and Decimates Secondary Lymphoid Organs
Abstract
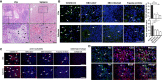
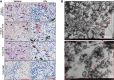
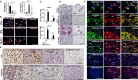
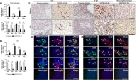
While lymphocytopenia is a common characteristic of coronavirus disease 2019 (COVID-19), the mechanisms responsible for this lymphocyte depletion are unclear. Here, we retrospectively reviewed the clinical and immunological data from 18 fatal COVID-19 cases, results showed that these patients had severe lymphocytopenia, together with high serum levels of inflammatory cytokines (IL-6, IL-8 and IL-10), and elevation of many other mediators in routine laboratory tests, including C-reactive protein, lactate dehydrogenase, α-hydroxybutyrate dehydrogenase and natriuretic peptide type B. The spleens and hilar lymph nodes (LNs) from six additional COVID-19 patients with post-mortem examinations were also collected, histopathologic detection showed that both organs manifested severe tissue damage and lymphocyte apoptosis in these six cases. In situ hybridization assays illustrated that SARS-CoV-2 viral RNA accumulates in these tissues, and transmission electronic microscopy confirmed that coronavirus-like particles were visible in the LNs. SARS-CoV-2 Spike and Nucleocapsid protein (NP) accumulated in the spleens and LNs, and the NP antigen restricted in angiotensin-converting enzyme 2 (ACE2) positive macrophages and dendritic cells (DCs). Furthermore, SARS-CoV-2 triggered the transcription of Il6, Il8 and Il1b genes in infected primary macrophages and DCs in vitro, and SARS-CoV-2-NP+ macrophages and DCs also manifested high levels of IL-6 and IL-1β, which might directly decimate human spleens and LNs and subsequently lead to lymphocytopenia in vivo. Collectively, these results demonstrated that SARS-CoV-2 induced lymphocytopenia by promoting systemic inflammation and direct neutralization in human spleen and LNs.
Keywords: COVID-19; SARS-CoV-2; dendritic cells; lymph nodes; lymphocytopenia; macrophages; spleen.
Copyright © 2021 Xiang, Feng, Diao, Tu, Qiao, Yang, Zhang, Wang, Wang, Wang, Liu, Wang, Liu, Chen, Wu and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Quantitative SARS-CoV-2 Serology in Children With Multisystem Inflammatory Syndrome (MIS-C).Pediatrics. 2020 Dec;146(6):e2020018242. doi: 10.1542/peds.2020-018242. Epub 2020 Sep 2. Pediatrics. 2020. PMID: 32879033
-
Coronavirus-19 (SARS-CoV-2) induces acute severe lung inflammation via IL-1 causing cytokine storm in COVID-19: a promising inhibitory strategy.J Biol Regul Homeost Agents. 2020 Nov-Dec;34(6):1971-1975. doi: 10.23812/20-1-E. J Biol Regul Homeost Agents. 2020. PMID: 33016027
-
Emerging Role of Platelet-Endothelium Interactions in the Pathogenesis of Severe SARS-CoV-2 Infection-Associated Myocardial Injury.Front Immunol. 2022 Feb 4;13:776861. doi: 10.3389/fimmu.2022.776861. eCollection 2022. Front Immunol. 2022. PMID: 35185878 Free PMC article. Review.
-
Lymphopenia an important immunological abnormality in patients with COVID-19: Possible mechanisms.Scand J Immunol. 2021 Feb;93(2):e12967. doi: 10.1111/sji.12967. Epub 2020 Sep 14. Scand J Immunol. 2021. PMID: 32875598 Review.
-
CD8+ T cells specific for an immunodominant SARS-CoV-2 nucleocapsid epitope display high naive precursor frequency and TCR promiscuity.Immunity. 2021 May 11;54(5):1066-1082.e5. doi: 10.1016/j.immuni.2021.04.009. Epub 2021 Apr 15. Immunity. 2021. PMID: 33951417 Free PMC article.
Cited by
-
Advancing insights in critical COVID-19: unraveling lymphopenia through propensity score matching - Findings from the Multicenter LYMPH-COVID Study.Crit Care Sci. 2024 Sep 27;36:e20240236en. doi: 10.62675/2965-2774.20240236-en. eCollection 2024. Crit Care Sci. 2024. PMID: 39356899 Free PMC article.
-
A comparison between virus- versus patients-centred therapeutic attempts to reduce COVID-19 mortality.Emerg Microbes Infect. 2021 Dec;10(1):2256-2263. doi: 10.1080/22221751.2021.2006579. Emerg Microbes Infect. 2021. PMID: 34783636 Free PMC article. Review.
-
Alterations in Circulating Monocytes Predict COVID-19 Severity and Include Chromatin Modifications Still Detectable Six Months after Recovery.Biomedicines. 2021 Sep 17;9(9):1253. doi: 10.3390/biomedicines9091253. Biomedicines. 2021. PMID: 34572439 Free PMC article.
-
Monocyte-driven atypical cytokine storm and aberrant neutrophil activation as key mediators of COVID-19 disease severity.Nat Commun. 2021 Jul 5;12(1):4117. doi: 10.1038/s41467-021-24360-w. Nat Commun. 2021. PMID: 34226537 Free PMC article.
-
Detection of SARS-CoV-2 Viral Genome and Viral Nucleocapsid in Various Organs and Systems.Int J Mol Sci. 2024 May 25;25(11):5755. doi: 10.3390/ijms25115755. Int J Mol Sci. 2024. PMID: 38891942 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

