Enkephalin release from VIP interneurons in the hippocampal CA2/3a region mediates heterosynaptic plasticity and social memory
- PMID: 33990774
- PMCID: PMC8590711
- DOI: 10.1038/s41380-021-01124-y
Enkephalin release from VIP interneurons in the hippocampal CA2/3a region mediates heterosynaptic plasticity and social memory
Abstract
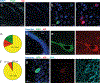
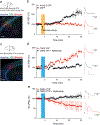
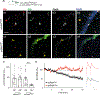
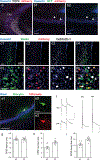
The hippocampus contains a diverse array of inhibitory interneurons that gate information flow through local cortico-hippocampal circuits to regulate memory storage. Although most studies of interneurons have focused on their role in fast synaptic inhibition mediated by GABA release, different classes of interneurons express unique sets of neuropeptides, many of which have been shown to exert powerful effects on neuronal function and memory when applied pharmacologically. However, relatively little is known about whether and how release of endogenous neuropeptides from inhibitory cells contributes to their behavioral role in regulating memory formation. Here we report that vasoactive intestinal peptide (VIP)-expressing interneurons participate in social memory storage by enhancing information transfer from hippocampal CA3 pyramidal neurons to CA2 pyramidal neurons. Notably, this action depends on release of the neuropeptide enkephalin from VIP neurons, causing long-term depression of feedforward inhibition onto CA2 pyramidal cells. Moreover, VIP neuron activity in the CA2 region is increased selectively during exploration of a novel conspecific. Our findings, thus, enhance our appreciation of how GABAergic neurons can regulate synaptic plasticity and mnemonic behavior by demonstrating that such actions can be mediated by release of a specific neuropeptide, rather than through classic fast inhibitory transmission.
© 2021. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Hypothalamic Supramammillary Nucleus Selectively Excites Hippocampal CA3 Interneurons to Suppress CA3 Pyramidal Neuron Activity.J Neurosci. 2023 Jun 21;43(25):4612-4624. doi: 10.1523/JNEUROSCI.1910-22.2023. Epub 2023 Apr 28. J Neurosci. 2023. PMID: 37117012 Free PMC article.
-
Input-Specific Synaptic Location and Function of the α5 GABAA Receptor Subunit in the Mouse CA1 Hippocampal Neurons.J Neurosci. 2019 Jan 30;39(5):788-801. doi: 10.1523/JNEUROSCI.0567-18.2018. Epub 2018 Dec 6. J Neurosci. 2019. PMID: 30523065 Free PMC article.
-
Activation of muscarinic receptors by ACh release in hippocampal CA1 depolarizes VIP but has varying effects on parvalbumin-expressing basket cells.J Physiol. 2015 Jan 1;593(1):197-215. doi: 10.1113/jphysiol.2014.277814. Epub 2014 Nov 28. J Physiol. 2015. PMID: 25556796 Free PMC article.
-
Inhibitory circuits in fear memory and fear-related disorders.Front Neural Circuits. 2023 Mar 23;17:1122314. doi: 10.3389/fncir.2023.1122314. eCollection 2023. Front Neural Circuits. 2023. PMID: 37035504 Free PMC article. Review.
-
Synaptic plasticity in hippocampal interneurons? A commentary.Can J Physiol Pharmacol. 1997 May;75(5):488-94. Can J Physiol Pharmacol. 1997. PMID: 9250382 Review.
Cited by
-
Reduced Cholecystokinin-Expressing Interneuron Input Contributes to Disinhibition of the Hippocampal CA2 Region in a Mouse Model of Temporal Lobe Epilepsy.J Neurosci. 2023 Oct 11;43(41):6930-6949. doi: 10.1523/JNEUROSCI.2091-22.2023. Epub 2023 Aug 29. J Neurosci. 2023. PMID: 37643861 Free PMC article.
-
Divergent opioid-mediated suppression of inhibition between hippocampus and neocortex across species and development.bioRxiv [Preprint]. 2024 Nov 2:2024.01.20.576455. doi: 10.1101/2024.01.20.576455. bioRxiv. 2024. PMID: 38313283 Free PMC article. Preprint.
-
The life and times of endogenous opioid peptides: Updated understanding of synthesis, spatiotemporal dynamics, and the clinical impact in alcohol use disorder.Neuropharmacology. 2023 Mar 1;225:109376. doi: 10.1016/j.neuropharm.2022.109376. Epub 2022 Dec 11. Neuropharmacology. 2023. PMID: 36516892 Free PMC article. Review.
-
Neural activation associated with outgroup helping in adolescent rats.iScience. 2022 May 16;25(6):104412. doi: 10.1016/j.isci.2022.104412. eCollection 2022 Jun 17. iScience. 2022. PMID: 35663035 Free PMC article.
-
Convergent, functionally independent signaling by mu and delta opioid receptors in hippocampal parvalbumin interneurons.Elife. 2021 Nov 17;10:e69746. doi: 10.7554/eLife.69746. Elife. 2021. PMID: 34787079 Free PMC article.
References
-
- Letzkus JJ, Wolff SBE, Meyer EMM, Tovote P, Courtin J, Herry C, et al. A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature. 2011;480:331–335. - PubMed
-
- Letzkus JJ, Wolff SBE, Lüthi A. Disinhibition, a Circuit Mechanism for Associative Learning and Memory. Neuron. 2015;88:264–276. - PubMed
-
- Krabbe S, Paradiso E, D’Aquin S, Bitterman Y, Courtin J, Xu C, et al. Adaptive disinhibitory gating by VIP interneurons permits associative learning. Nat Neurosci. 2019;22:1834–1843. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

