Cephalomannine inhibits hypoxia-induced cellular function via the suppression of APEX1/HIF-1α interaction in lung cancer
- PMID: 33990544
- PMCID: PMC8121842
- DOI: 10.1038/s41419-021-03771-z
Cephalomannine inhibits hypoxia-induced cellular function via the suppression of APEX1/HIF-1α interaction in lung cancer
Abstract
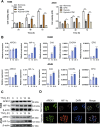
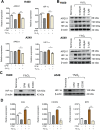
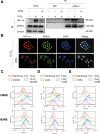
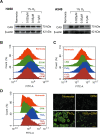
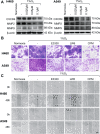
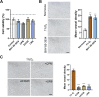
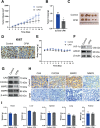
Lung cancer (LC) is one of the leading causes of cancer-related death. As one of the key features of tumor microenvironment, hypoxia conditions are associated with poor prognosis in LC patients. Upregulation of hypoxic-induced factor-1α (HIF-1α) leads to the activation of various factors that contribute to the increased drug resistance, proliferation, and migration of tumor cells. Apurinic/apyrimidinic endonuclease-1 (APEX1) is a multi-functional protein that regulates several transcription factors, including HIF-1α, that contribute to tumor growth, oxidative stress responses, and DNA damage. In this study, we explored the mechanisms underlying cell responses to hypoxia and modulation of APEX1, which regulate HIF-1α and downstream pathways. We found that hypoxia-induced APEX1/HIF-1α pathways regulate several key cellular functions, including reactive oxygen species (ROS) production, carbonic anhydrase 9 (CA9)-mediated intracellular pH, migration, and angiogenesis. Cephalomannine (CPM), a natural compound, exerted inhibitory effects in hypoxic LC cells via the inhibition of APEX1/HIF-1α interaction in vitro and in vivo. CPM can significantly inhibit cell viability, ROS production, intracellular pH, and migration in hypoxic LC cells as well as angiogenesis of HUVECs under hypoxia through the inhibition of APEX1/HIF-1α interaction. Taken together, CPM could be considered as a promising compound for LC treatment.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Molecular and functional evaluation of a novel HIF inhibitor, benzopyranyl 1,2,3-triazole compound.Oncotarget. 2017 Jan 31;8(5):7801-7813. doi: 10.18632/oncotarget.13955. Oncotarget. 2017. PMID: 27999195 Free PMC article.
-
Blocking HIF signaling via novel inhibitors of CA9 and APE1/Ref-1 dramatically affects pancreatic cancer cell survival.Sci Rep. 2018 Sep 13;8(1):13759. doi: 10.1038/s41598-018-32034-9. Sci Rep. 2018. PMID: 30214007 Free PMC article.
-
The Apoptotic Effect of HIF-1α Inhibition Combined with Glucose plus Insulin Treatment on Gastric Cancer under Hypoxic Conditions.PLoS One. 2015 Sep 4;10(9):e0137257. doi: 10.1371/journal.pone.0137257. eCollection 2015. PLoS One. 2015. PMID: 26339797 Free PMC article.
-
Modulation of carbonic anhydrase 9 (CA9) in human brain cancer.Curr Pharm Des. 2010;16(29):3288-99. doi: 10.2174/138161210793429788. Curr Pharm Des. 2010. PMID: 20819065 Review.
-
Melatonin and the von Hippel-Lindau/HIF-1 oxygen sensing mechanism: A review.Biochim Biophys Acta. 2016 Apr;1865(2):176-83. doi: 10.1016/j.bbcan.2016.02.004. Epub 2016 Feb 17. Biochim Biophys Acta. 2016. PMID: 26899267 Review.
Cited by
-
Identification of new microtubule small-molecule inhibitors and microtubule-associated genes against triple negative breast cancer.Am J Cancer Res. 2024 Apr 15;14(4):1545-1560. doi: 10.62347/LYDF1241. eCollection 2024. Am J Cancer Res. 2024. PMID: 38726264 Free PMC article.
-
The Role of Hypoxia-Inducible Factor-1 Alpha in Renal Disease.Molecules. 2022 Oct 28;27(21):7318. doi: 10.3390/molecules27217318. Molecules. 2022. PMID: 36364144 Free PMC article. Review.
-
Oxygen-evolving photosynthetic cyanobacteria for 2D bismuthene radiosensitizer-enhanced cancer radiotherapy.Bioact Mater. 2022 Jan 20;17:276-288. doi: 10.1016/j.bioactmat.2022.01.014. eCollection 2022 Nov. Bioact Mater. 2022. PMID: 35386463 Free PMC article.
-
Advancements in the Cultivation, Active Components, and Pharmacological Activities of Taxus mairei.Molecules. 2024 Mar 2;29(5):1128. doi: 10.3390/molecules29051128. Molecules. 2024. PMID: 38474640 Free PMC article. Review.
-
The Use of Breath Analysis in the Management of Lung Cancer: Is It Ready for Primetime?Curr Oncol. 2022 Sep 30;29(10):7355-7378. doi: 10.3390/curroncol29100578. Curr Oncol. 2022. PMID: 36290855 Free PMC article. Review.
References
-
- Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.10.3322/caac.21660 (2021). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

