TLR7 and IL-6 differentially regulate the effects of rotarod exercise on the transcriptomic profile and neurogenesis to influence anxiety and memory
- PMID: 33981972
- PMCID: PMC8082089
- DOI: 10.1016/j.isci.2021.102384
TLR7 and IL-6 differentially regulate the effects of rotarod exercise on the transcriptomic profile and neurogenesis to influence anxiety and memory
Abstract
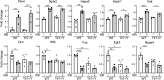
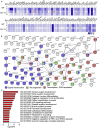
Voluntary exercise is well known to benefit brain performance. In contrast, forced exercise induces inflammation-related stress responses and may cause psychiatric disorders. Here, we unexpectedly found that rotarod testing, a frequently applied assay for evaluating rodent motor coordination, induces anxiety and alters spatial learning/memory performance of mice. Rotarod testing upregulated genes involved in the unfolded protein response and stress responses and downregulated genes associated with neurogenesis and neuronal differentiation. It impacts two downstream pathways. The first is the IL-6-dependent pathway, which mediates rotarod-induced anxiety. The second is the Toll-like receptor 7 (TLR7)-dependent pathway, which is involved in the effect of rotarod exercise on gene expression and its impact on contextual learning and memory of mice. Thus, although rotarod exercise does not induce systemic inflammation, it influences innate immunity-related responses in the brain, controls gene expression and, consequently, regulates anxiety and contextual learning and memory.
Keywords: Immunology; Molecular Neuroscience; Transcriptomics.
© 2021 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Tlr7 deletion alters expression profiles of genes related to neural function and regulates mouse behaviors and contextual memory.Brain Behav Immun. 2018 Aug;72:101-113. doi: 10.1016/j.bbi.2018.06.006. Epub 2018 Jun 7. Brain Behav Immun. 2018. PMID: 29885943
-
Intact neurogenesis is required for benefits of exercise on spatial memory but not motor performance or contextual fear conditioning in C57BL/6J mice.Neuroscience. 2008 Sep 9;155(4):1048-58. doi: 10.1016/j.neuroscience.2008.06.051. Epub 2008 Jul 1. Neuroscience. 2008. PMID: 18664375
-
Mouse genetic differences in voluntary wheel running, adult hippocampal neurogenesis and learning on the multi-strain-adapted plus water maze.Behav Brain Res. 2015 Mar 1;280:62-71. doi: 10.1016/j.bbr.2014.11.030. Epub 2014 Nov 27. Behav Brain Res. 2015. PMID: 25435316 Free PMC article.
-
Effects of forced exercise on spatial memory and cytochrome c oxidase activity in aged rats.Brain Res. 2013 Mar 28;1502:20-9. doi: 10.1016/j.brainres.2012.12.036. Epub 2013 Jan 31. Brain Res. 2013. PMID: 23375841
-
Beyond defense: regulation of neuronal morphogenesis and brain functions via Toll-like receptors.J Biomed Sci. 2019 Nov 4;26(1):90. doi: 10.1186/s12929-019-0584-z. J Biomed Sci. 2019. PMID: 31684953 Free PMC article. Review.
Cited by
-
Treadmill exercise modulates the medial prefrontal-amygdala neural circuit to improve the resilience against chronic restraint stress.Commun Biol. 2023 Jun 9;6(1):624. doi: 10.1038/s42003-023-05003-w. Commun Biol. 2023. PMID: 37296310 Free PMC article.
-
Empagliflozin attenuates neurodegeneration through antioxidant, anti-inflammatory, and modulation of α-synuclein and Parkin levels in rotenone-induced Parkinson's disease in rats.Saudi Pharm J. 2022 Jun;30(6):863-873. doi: 10.1016/j.jsps.2022.03.005. Epub 2022 Mar 16. Saudi Pharm J. 2022. PMID: 35812142 Free PMC article.
-
Sex bias in social deficits, neural circuits and nutrient demand in Cttnbp2 autism models.Brain. 2023 Jun 1;146(6):2612-2626. doi: 10.1093/brain/awac429. Brain. 2023. PMID: 36385662 Free PMC article.
References
-
- Akira S., Sato S. Toll-like receptors and their signaling mechanisms. Scand. J. Infect. Dis. 2003;35:555–562. - PubMed
-
- Bettio L., Thacker J.S., Hutton C., Christie B.R. Modulation of synaptic plasticity by exercise. Int. Rev. Neurobiol. 2019;147:295–322. - PubMed
-
- Burgueño J.F., Abreu M.T. Epithelial Toll-like receptors and their role in gut homeostasis and disease. Nat. Rev. Gastroenterol. Hepatol. 2020;17:263–278. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

