Prevalence of antibody positivity to SARS-CoV-2 following the first peak of infection in England: Serial cross-sectional studies of 365,000 adults
- PMID: 33969335
- PMCID: PMC8088780
- DOI: 10.1016/j.lanepe.2021.100098
Prevalence of antibody positivity to SARS-CoV-2 following the first peak of infection in England: Serial cross-sectional studies of 365,000 adults
Abstract
Background: The time-concentrated nature of the first wave of the COVID-19 epidemic in England in March and April 2020 provides a natural experiment to measure changes in antibody positivity at the population level before onset of the second wave and initiation of the vaccination programme.
Methods: Three cross-sectional national surveys with non-overlapping random samples of the population in England undertaken between late June and September 2020 (REACT-2 study). 365,104 adults completed questionnaires and self-administered lateral flow immunoassay (LFIA) tests for IgG against SARS-CoV-2.
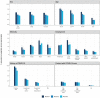
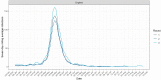
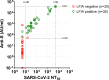
Findings: Overall, 17,576 people had detectable antibodies, a prevalence of 4.9% (95% confidence intervals 4.9, 5.0) when adjusted for test characteristics and weighted to the adult population of England. The prevalence declined from 6.0% (5.8, 6.1), to 4.8% (4.7, 5.0) and 4.4% (4.3, 4.5), over the three rounds of the study a difference of -26.5% (-29.0, -23.8). The highest prevalence and smallest overall decline in positivity was in the youngest age group (18-24 years) at -14.9% (-21.6, -8.1), and lowest prevalence and largest decline in the oldest group (>74 years) at -39.0% (-50.8, -27.2). The decline from June to September 2020 was largest in those who did not report a history of COVID-19 at -64.0% (-75.6, -52.3), compared to -22.3% (-27.0, -17.7) in those with SARS-CoV-2 infection confirmed on PCR.
Interpretation: A large proportion of the population remained susceptible to SARS-CoV-2 infection in England based on naturally acquired immunity from the first wave. Widespread vaccination is needed to confer immunity and control the epidemic at population level.
Funding: This work was funded by the Department of Health and Social Care in England.
© 2021 The Author(s).
Conflict of interest statement
CAD reports grants from UK Medical Research Council, grants from UK NIHR, during the conduct of the study; HW and PE report grants from the Department of Health and Social Care during the conduct of this study. The remaining authors have nothing to disclose.
Figures
Similar articles
-
Design and Implementation of a National Program to Monitor the Prevalence of SARS-CoV-2 IgG Antibodies in England Using Self-Testing: The REACT-2 Study.Am J Public Health. 2023 Nov;113(11):1201-1209. doi: 10.2105/AJPH.2023.307381. Epub 2023 Sep 21. Am J Public Health. 2023. PMID: 37733993 Free PMC article.
-
SARS-CoV-2 infection and vaccine effectiveness in England (REACT-1): a series of cross-sectional random community surveys.Lancet Respir Med. 2022 Apr;10(4):355-366. doi: 10.1016/S2213-2600(21)00542-7. Epub 2022 Jan 24. Lancet Respir Med. 2022. PMID: 35085490 Free PMC article.
-
COVID-19 lateral flow IgG seropositivity and serum neutralising antibody responses after primary and booster vaccinations in Chile: a cross-sectional study.Lancet Microbe. 2023 Mar;4(3):e149-e158. doi: 10.1016/S2666-5247(22)00290-7. Epub 2023 Jan 27. Lancet Microbe. 2023. PMID: 36716754 Free PMC article.
-
Authors' response: Occupation and SARS-CoV-2 infection risk among workers during the first pandemic wave in Germany: potential for bias.Scand J Work Environ Health. 2022 Sep 1;48(7):588-590. doi: 10.5271/sjweh.4061. Epub 2022 Sep 25. Scand J Work Environ Health. 2022. PMID: 36153787 Free PMC article. Review.
-
Rapid, point-of-care antigen tests for diagnosis of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2022 Jul 22;7(7):CD013705. doi: 10.1002/14651858.CD013705.pub3. Cochrane Database Syst Rev. 2022. PMID: 35866452 Free PMC article. Review.
Cited by
-
COVID-19 seroprevalence cohort survey among health care workers and their household members in Kinshasa, DR Congo, 2020-2022.J Health Popul Nutr. 2024 Jun 1;43(1):74. doi: 10.1186/s41043-024-00536-0. J Health Popul Nutr. 2024. PMID: 38824595 Free PMC article.
-
Contribution of infection and vaccination to population-level seroprevalence through two COVID waves in Tamil Nadu, India.Sci Rep. 2024 Jan 24;14(1):2091. doi: 10.1038/s41598-023-50338-3. Sci Rep. 2024. PMID: 38267448 Free PMC article.
-
Novel machine-learning analysis of SARS-CoV-2 infection in a subclinical nonhuman primate model using radiomics and blood biomarkers.Sci Rep. 2023 Nov 10;13(1):19607. doi: 10.1038/s41598-023-46694-9. Sci Rep. 2023. PMID: 37950044 Free PMC article.
-
Design and Implementation of a National Program to Monitor the Prevalence of SARS-CoV-2 IgG Antibodies in England Using Self-Testing: The REACT-2 Study.Am J Public Health. 2023 Nov;113(11):1201-1209. doi: 10.2105/AJPH.2023.307381. Epub 2023 Sep 21. Am J Public Health. 2023. PMID: 37733993 Free PMC article.
-
SARS-CoV-2 rapid antibody test results and subsequent risk of hospitalisation and death in 361,801 people.Nat Commun. 2023 Aug 16;14(1):4957. doi: 10.1038/s41467-023-40643-w. Nat Commun. 2023. PMID: 37587102 Free PMC article.
References
-
- Patel MM, Thornburg NJ, Stubblefield WB, Talbot HK, Coughlin MM, Feldstein LR. Change in antibodies to SARS-CoV-2 Over 60 days among health care personnel in Nashville, Tennessee. JAMA. 2020 https://jamanetwork.com/journals/jama/fullarticle/2770928 [Internet]Sep 17 [cited 2020 Oct 3]; Available from: - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

