LINC-PINT impedes DNA repair and enhances radiotherapeutic response by targeting DNA-PKcs in nasopharyngeal cancer
- PMID: 33963177
- PMCID: PMC8105365
- DOI: 10.1038/s41419-021-03728-2
LINC-PINT impedes DNA repair and enhances radiotherapeutic response by targeting DNA-PKcs in nasopharyngeal cancer
Abstract
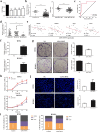
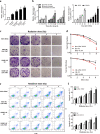
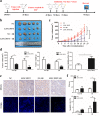
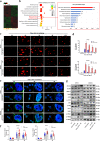
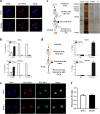
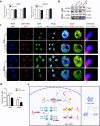
Radioresistance continues to be the leading cause of recurrence and metastasis in nasopharyngeal cancer. Long noncoding RNAs are emerging as regulators of DNA damage and radioresistance. LINC-PINT was originally identified as a tumor suppressor in various cancers. In this study, LINC-PINT was significantly downregulated in nasopharyngeal cancer tissues than in rhinitis tissues, and low LINC-PINT expressions showed poorer prognosis in patients who received radiotherapy. We further identified a functional role of LINC-PINT in inhibiting the malignant phenotypes and sensitizing cancer cells to irradiation in vitro and in vivo. Mechanistically, LINC-PINT was responsive to DNA damage, inhibiting DNA damage repair through ATM/ATR-Chk1/Chk2 signaling pathways. Moreover, LINC-PINT increased radiosensitivity by interacting with DNA-dependent protein kinase catalytic subunit (DNA-PKcs) and negatively regulated the expression and recruitment of DNA-PKcs. Therefore, these findings collectively support the possibility that LINC-PINT serves as an attractive target to overcome radioresistance in NPC.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
LINC-PINT plays an anti-tumor role in nasopharyngeal carcinoma by binding to XRCC6 and affecting its function.Pathol Res Pract. 2024 Aug;260:155460. doi: 10.1016/j.prp.2024.155460. Epub 2024 Jul 18. Pathol Res Pract. 2024. PMID: 39032384
-
LINC-PINT suppresses tumour cell proliferation, migration and invasion through targeting miR-374a-5p in ovarian cancer.Cell Biochem Funct. 2020 Dec;38(8):1089-1099. doi: 10.1002/cbf.3565. Epub 2020 Jul 7. Cell Biochem Funct. 2020. PMID: 32638404
-
Long noncoding RNA LINC-PINT is inhibited in gastric cancer and predicts poor survival.J Cell Biochem. 2019 Jun;120(6):9594-9600. doi: 10.1002/jcb.28236. Epub 2018 Dec 19. J Cell Biochem. 2019. PMID: 30569513
-
Long intragenic non-coding RNA p53-induced transcript (LINC-PINT) as a novel prognosis indicator and therapeutic target in cancer.Biomed Pharmacother. 2021 Nov;143:112127. doi: 10.1016/j.biopha.2021.112127. Epub 2021 Aug 30. Biomed Pharmacother. 2021. PMID: 34474342 Review.
-
PINTology: A short history of the lncRNA LINC-PINT in different diseases.Wiley Interdiscip Rev RNA. 2022 Jul;13(4):e1705. doi: 10.1002/wrna.1705. Epub 2022 Jan 12. Wiley Interdiscip Rev RNA. 2022. PMID: 35019222 Review.
Cited by
-
Role of long non-coding RNA in chemoradiotherapy resistance of nasopharyngeal carcinoma.Front Oncol. 2024 Feb 29;14:1346413. doi: 10.3389/fonc.2024.1346413. eCollection 2024. Front Oncol. 2024. PMID: 38487724 Free PMC article. Review.
-
Clinical implications of lncRNA LINC-PINT in cancer.Front Mol Biosci. 2023 Mar 17;10:1097694. doi: 10.3389/fmolb.2023.1097694. eCollection 2023. Front Mol Biosci. 2023. PMID: 37006616 Free PMC article.
-
Mechanisms of traditional Chinese medicine overcoming of radiotherapy resistance in breast cancer.Front Oncol. 2024 Jun 27;14:1388750. doi: 10.3389/fonc.2024.1388750. eCollection 2024. Front Oncol. 2024. PMID: 38993643 Free PMC article.
-
m6A- and m5C- modified lncRNAs orchestrate the prognosis in cutaneous melanoma and m6A- modified LINC00893 regulates cutaneous melanoma cell metastasis.Skin Res Technol. 2024 Jul;30(7):e13842. doi: 10.1111/srt.13842. Skin Res Technol. 2024. PMID: 38965799 Free PMC article.
-
Comprehensive analysis of cuproptosis-related lncRNAs in the prognosis and therapy response of patients with bladder cancer.Ann Transl Med. 2022 Nov;10(22):1232. doi: 10.21037/atm-22-5294. Ann Transl Med. 2022. PMID: 36544685 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

