Neoadjuvant immunoradiotherapy results in high rate of complete pathological response and clinical to pathological downstaging in locally advanced head and neck squamous cell carcinoma
- PMID: 33963014
- PMCID: PMC8108690
- DOI: 10.1136/jitc-2021-002485
Neoadjuvant immunoradiotherapy results in high rate of complete pathological response and clinical to pathological downstaging in locally advanced head and neck squamous cell carcinoma
Abstract
Background: Checkpoint inhibitors targeting programmed death receptor-1 (PD-1) have been tested in the neoadjuvant setting for the treatment of locoregionally advanced head and neck squamous cell carcinoma (HNSCC); however, response rates are modest. We hypothesized that adding stereotactic body radiation therapy (SBRT) to anti-PD-1 would be safe prior to definitive surgical resection and would enhance pathological response compared with historical cohorts of patients with locoregionally advanced HNSCC treated with checkpoint inhibitor alone.
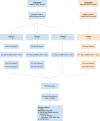
Methods: The Neoadjuvant Immuno-Radiotherapy Trial was an investigator-initiated single institution phase Ib clinical trial that enrolled patients with previously untreated locally advanced HPV-positive and HPV-negative HNSCC between 2018 and 2019. Eligible patients were treated with neoadjuvant SBRT at a total dose of either 40 Gy in 5 fractions or 24 Gy in 3 fractions, delivered in a 1-week timespan, with or without nivolumab, prior to definitive surgical resection. Patients were then planned for treatment with adjuvant nivolumab for 3 months. The primary safety endpoint was unplanned delay in surgery considered to be at least possibly related to neoadjuvant treatment. The primary efficacy endpoints included pathological complete response (pCR), major pathological response (mPR), and the rate of clinical to pathological downstaging after neoadjuvant treatment.
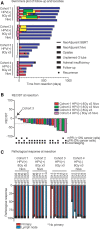
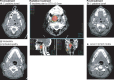
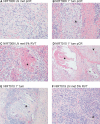
Results: Twenty-one patients underwent neoadjuvant treatment, which was well tolerated and did not delay surgery, thus meeting the primary endpoint. Tissue responses were characterized by robust inflammatory infiltrates in the regression bed, plasma cells and cholesterol clefts. Among the entire study group, the mPR and pCR rate was 86% and 67%, respectively. Clinical to pathological downstaging occurred in 90% of the patients treated.
Conclusion: These data demonstrate that radiation delivered only to the gross tumor volume combined with immunotherapy was safe, resulted in a high rate of mPR and should be further evaluated as a locally focused neoadjuvant therapy for patients with head and neck cancer.
Trial registration number: This study is registered with clinicaltrials.gov (NCT03247712) and is active, but closed to patient accrual.
Keywords: combination; drug therapy; head and neck neoplasms; immunotherapy; radioimmunotherapy.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: The Earle A. Chiles Research Institute receives research funding from Bristol-Myers Squibb, none of which was used in the conduct of this trial; and AstraZeneca. RSL reports grants from Bristol Myers Squibb, personal fees from Merck, personal fees from Sanofi, personal fees from Regeneron, personal fees from Oncolys, outside the submitted work; MRC reports research grants from Jounce Therapeutics, grants from Nanobiotix, outside the submitted work. KHY reports non-financial support and material support for clinical trial from Eli Lilly; sponsored project agreement and institutional grant support from Bristol-Myers Squibb; and other support from AstraZeneca, outside the submitted work; CBB reports a consultant/advisory relationship with PrimeVax and Bristol-Myers Squibb; stock ownership and scientific board membership in PrimeVax and BioAI; and holds a patent on image processing systems and methods for displaying multiple images of a biological specimen that was outside the submitted work; WJU reports grants from Bristol-Myers Squibb, grants and other institutional support from Galectin Therapeutics, grants and other institutional support from Astra Zeneca, grants and other institutional support from Prometheus, grants and other institutional support from Merck, outside the submitted work; MJG reports grants from Bristol Myers Squibb, grants from Mavupharma, outside the submitted work; RBB reports grants and personal fees from Bristol Myers Squibb, grants and personal fees from Merck, personal fees from Regeneron, outside the submitted work. All other authors declare no competing interests.
Figures
Similar articles
-
Neoadjuvant nivolumab for patients with resectable HPV-positive and HPV-negative squamous cell carcinomas of the head and neck in the CheckMate 358 trial.J Immunother Cancer. 2021 Jun;9(6):e002568. doi: 10.1136/jitc-2021-002568. J Immunother Cancer. 2021. PMID: 34083421 Free PMC article. Clinical Trial.
-
Neoadjuvant immunotherapy with nivolumab and ipilimumab induces major pathological responses in patients with head and neck squamous cell carcinoma.Nat Commun. 2021 Dec 22;12(1):7348. doi: 10.1038/s41467-021-26472-9. Nat Commun. 2021. PMID: 34937871 Free PMC article. Clinical Trial.
-
Phase III study of nivolumab alone or combined with ipilimumab as immunotherapy versus standard of care in resectable head and neck squamous cell carcinoma.Future Oncol. 2020 Dec;16(36):3035-3043. doi: 10.2217/fon-2020-0595. Epub 2020 Sep 9. Future Oncol. 2020. PMID: 32902312 Clinical Trial.
-
Anti-PD-(L)1-Based Neoadjuvant Therapy in Head and Neck Carcinoma: a Meta-analysis of Prospective Clinical Trials.Otolaryngol Head Neck Surg. 2024 Nov;171(5):1321-1340. doi: 10.1002/ohn.867. Epub 2024 Jun 29. Otolaryngol Head Neck Surg. 2024. PMID: 38943451
-
Advancements in neoadjuvant immune checkpoint inhibitor therapy for locally advanced head and neck squamous Carcinoma: A narrative review.Int Immunopharmacol. 2024 Jun 15;134:112200. doi: 10.1016/j.intimp.2024.112200. Epub 2024 May 13. Int Immunopharmacol. 2024. PMID: 38744175 Review.
Cited by
-
Current status of neoadjuvant immunotherapy for the treatment of gastric cancer.Clin Transl Oncol. 2024 Sep;26(9):2097-2108. doi: 10.1007/s12094-024-03437-0. Epub 2024 Mar 20. Clin Transl Oncol. 2024. PMID: 38504071 Review.
-
The impact of elective cervical lymph node treatment on the tumour immune response in head and neck squamous cell carcinoma: time for a change in treatment strategy?BJC Rep. 2024 Sep 9;2(1):68. doi: 10.1038/s44276-024-00095-1. BJC Rep. 2024. PMID: 39516703 Free PMC article. Review.
-
Lymphatic-preserving treatment sequencing with immune checkpoint inhibition unleashes cDC1-dependent antitumor immunity in HNSCC.Nat Commun. 2022 Jul 25;13(1):4298. doi: 10.1038/s41467-022-31941-w. Nat Commun. 2022. PMID: 35879302 Free PMC article.
-
The immunogenic radiation and new players in immunotherapy and targeted therapy for head and neck cancer.Front Oral Health. 2023 Jul 11;4:1180869. doi: 10.3389/froh.2023.1180869. eCollection 2023. Front Oral Health. 2023. PMID: 37496754 Free PMC article. Review.
-
Emerging evidence for adapting radiotherapy to immunotherapy.Nat Rev Clin Oncol. 2023 Aug;20(8):543-557. doi: 10.1038/s41571-023-00782-x. Epub 2023 Jun 6. Nat Rev Clin Oncol. 2023. PMID: 37280366 Review.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
