Co-targeting myelin inhibitors and CSPGs markedly enhances regeneration of GDNF-stimulated, but not conditioning-lesioned, sensory axons into the spinal cord
- PMID: 33942723
- PMCID: PMC8139830
- DOI: 10.7554/eLife.63050
Co-targeting myelin inhibitors and CSPGs markedly enhances regeneration of GDNF-stimulated, but not conditioning-lesioned, sensory axons into the spinal cord
Abstract
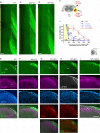
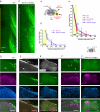
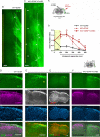
A major barrier to intraspinal regeneration after dorsal root (DR) injury is the DR entry zone (DREZ), the CNS/PNS interface. DR axons stop regenerating at the DREZ, even if regenerative capacity is increased by a nerve conditioning lesion. This potent blockade has long been attributed to myelin-associated inhibitors and (CSPGs), but incomplete lesions and conflicting reports have prevented conclusive agreement. Here, we evaluated DR regeneration in mice using novel strategies to facilitate complete lesions and analyses, selective tracing of proprioceptive and mechanoreceptive axons, and the first simultaneous targeting of Nogo/Reticulon-4, MAG, OMgp, CSPGs, and GDNF. Co-eliminating myelin inhibitors and CSPGs elicited regeneration of only a few conditioning-lesioned DR axons across the DREZ. Their absence, however, markedly and synergistically enhanced regeneration of GDNF-stimulated axons, highlighting the importance of sufficiently elevating intrinsic growth capacity. We also conclude that myelin inhibitors and CSPGs are not the primary mechanism stopping axons at the DREZ.
Keywords: DRG; Nogo; dorsal root injury; mouse; neuroscience; rhizotomy; sensory axon; spinal cord regeneration.
© 2021, Zhai et al.
Conflict of interest statement
JZ, HK, SH, MM, RY, SP, GS, YS No competing interests declared
Figures
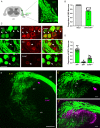


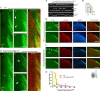

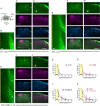


Similar articles
-
Deleting PTEN, but not SOCS3 or myelin inhibitors, robustly boosts BRAF-elicited intraspinal regeneration of peripheral sensory axons.bioRxiv [Preprint]. 2024 Sep 19:2024.09.18.613685. doi: 10.1101/2024.09.18.613685. bioRxiv. 2024. PMID: 39345461 Free PMC article. Preprint.
-
Spinal cord regeneration.Cell Transplant. 2014;23(4-5):573-611. doi: 10.3727/096368914X678427. Cell Transplant. 2014. PMID: 24816452 Review.
-
Postinjury Induction of Activated ErbB2 Selectively Hyperactivates Denervated Schwann Cells and Promotes Robust Dorsal Root Axon Regeneration.J Neurosci. 2017 Nov 8;37(45):10955-10970. doi: 10.1523/JNEUROSCI.0903-17.2017. Epub 2017 Oct 5. J Neurosci. 2017. PMID: 28982707 Free PMC article.
-
Live imaging of dorsal root axons after rhizotomy.J Vis Exp. 2011 Sep 1;(55):e3126. doi: 10.3791/3126. J Vis Exp. 2011. PMID: 21912366 Free PMC article.
-
Netrin-1 signaling for sensory axons: Involvement in sensory axonal development and regeneration.Cell Adh Migr. 2009 Apr-Jun;3(2):171-3. doi: 10.4161/cam.3.2.7837. Epub 2009 Apr 14. Cell Adh Migr. 2009. PMID: 19262170 Free PMC article. Review.
Cited by
-
Cytokine Signalling at the Microglial Penta-Partite Synapse.Int J Mol Sci. 2021 Dec 7;22(24):13186. doi: 10.3390/ijms222413186. Int J Mol Sci. 2021. PMID: 34947983 Free PMC article. Review.
-
Co-targeting B-RAF and PTEN Enables Sensory Axons to Regenerate Across and Beyond the Spinal Cord Injury.Front Mol Neurosci. 2022 Apr 26;15:891463. doi: 10.3389/fnmol.2022.891463. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35557554 Free PMC article.
-
Dorsal Root Injury-A Model for Exploring Pathophysiology and Therapeutic Strategies in Spinal Cord Injury.Cells. 2021 Aug 25;10(9):2185. doi: 10.3390/cells10092185. Cells. 2021. PMID: 34571835 Free PMC article. Review.
-
The Regulation of Spermatogonial Stem Cells in an Adult Testis by Glial Cell Line-Derived Neurotrophic Factor.Front Endocrinol (Lausanne). 2022 Jun 3;13:896390. doi: 10.3389/fendo.2022.896390. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35721702 Free PMC article. Review.
References
-
- Andrews MR, Czvitkovich S, Dassie E, Vogelaar CF, Faissner A, Blits B, Gage FH, ffrench-Constant C, Fawcett JW. Alpha9 integrin promotes neurite outgrowth on tenascin-C and enhances sensory axon regeneration. Journal of Neuroscience. 2009;29:5546–5557. doi: 10.1523/JNEUROSCI.0759-09.2009. - DOI - PMC - PubMed
-
- Blesch A, Lu P, Tsukada S, Alto LT, Roet K, Coppola G, Geschwind D, Tuszynski MH. Conditioning lesions before or after spinal cord injury recruit broad genetic mechanisms that sustain axonal regeneration: superiority to camp-mediated effects. Experimental Neurology. 2012;235:162–173. doi: 10.1016/j.expneurol.2011.12.037. - DOI - PMC - PubMed
-
- Busch SA, Horn KP, Cuascut FX, Hawthorne AL, Bai L, Miller RH, Silver J. Adult NG2+ cells are permissive to neurite outgrowth and stabilize sensory axons during macrophage-induced axonal dieback after spinal cord injury. Journal of Neuroscience. 2010;30:255–265. doi: 10.1523/JNEUROSCI.3705-09.2010. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

