A new non-aggregative splicing isoform of human Tau is decreased in Alzheimer's disease
- PMID: 33934221
- PMCID: PMC8217066
- DOI: 10.1007/s00401-021-02317-z
A new non-aggregative splicing isoform of human Tau is decreased in Alzheimer's disease
Abstract
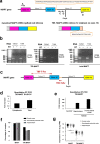
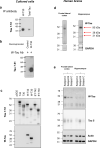
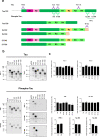
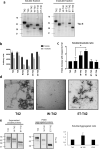
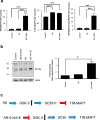
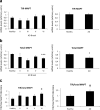
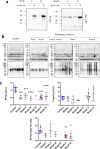
Tauopathies, including Alzheimer's disease (AD) and frontotemporal lobar degeneration with Tau pathology (FTLD-tau), are a group of neurodegenerative disorders characterized by Tau hyperphosphorylation. Post-translational modifications of Tau such as phosphorylation and truncation have been demonstrated to be an essential step in the molecular pathogenesis of these tauopathies. In this work, we demonstrate the existence of a new, human-specific truncated form of Tau generated by intron 12 retention in human neuroblastoma cells and, to a higher extent, in human RNA brain samples, using qPCR and further confirming the results on a larger database of human RNA-seq samples. Diminished protein levels of this new Tau isoform are found by Westernblotting in Alzheimer's patients' brains (Braak I n = 3; Braak II n = 6, Braak III n = 3, Braak IV n = 1, and Braak V n = 10, Braak VI n = 8) with respect to non-demented control subjects (n = 9), suggesting that the lack of this truncated isoform may play an important role in the pathology. This new Tau isoform exhibits similar post-transcriptional modifications by phosphorylation and affinity for microtubule binding, but more interestingly, is less prone to aggregate than other Tau isoforms. Finally, we present evidence suggesting this new Tau isoform could be linked to the inhibition of GSK3β, which would mediate intron 12 retention by modulating the serine/arginine rich splicing factor 2 (SRSF2). Our results show the existence of an important new isoform of Tau and suggest that further research on this less aggregation-prone Tau may help to develop future therapies for Alzheimer's disease and other tauopathies.
Keywords: Alternative splicing; Alzheimer’s disease; Intron retention; Tau; Tauopathies; Truncation.
Conflict of interest statement
The authors declare that they have no competing interests to disclose.
Figures
Similar articles
-
The Role of Tau Proteoforms in Health and Disease.Mol Neurobiol. 2023 Sep;60(9):5155-5166. doi: 10.1007/s12035-023-03387-8. Epub 2023 Jun 2. Mol Neurobiol. 2023. PMID: 37266762 Review.
-
Detection of Alzheimer's disease (AD) specific tau pathology with conformation-selective anti-tau monoclonal antibody in co-morbid frontotemporal lobar degeneration-tau (FTLD-tau).Acta Neuropathol Commun. 2019 Mar 4;7(1):34. doi: 10.1186/s40478-019-0687-5. Acta Neuropathol Commun. 2019. PMID: 30832741 Free PMC article.
-
Intron retention as a productive mechanism in human MAPT: RNA species generated by retention of intron 3.EBioMedicine. 2024 Feb;100:104953. doi: 10.1016/j.ebiom.2023.104953. Epub 2024 Jan 5. EBioMedicine. 2024. PMID: 38181704 Free PMC article.
-
Transactive response DNA-binding protein 43 (TDP-43) regulates alternative splicing of tau exon 10: Implications for the pathogenesis of tauopathies.J Biol Chem. 2017 Jun 23;292(25):10600-10612. doi: 10.1074/jbc.M117.783498. Epub 2017 May 9. J Biol Chem. 2017. PMID: 28487370 Free PMC article.
-
Tau alternative splicing in familial and sporadic tauopathies.Biochem Soc Trans. 2012 Aug;40(4):677-80. doi: 10.1042/BST20120091. Biochem Soc Trans. 2012. PMID: 22817715 Review.
Cited by
-
Tau truncation in the pathogenesis of Alzheimer's disease: a narrative review.Neural Regen Res. 2024 Jun 1;19(6):1221-1232. doi: 10.4103/1673-5374.385853. Epub 2023 Sep 22. Neural Regen Res. 2024. PMID: 37905868 Free PMC article.
-
The Role of Tau Proteoforms in Health and Disease.Mol Neurobiol. 2023 Sep;60(9):5155-5166. doi: 10.1007/s12035-023-03387-8. Epub 2023 Jun 2. Mol Neurobiol. 2023. PMID: 37266762 Review.
-
Posttranscriptional regulation of neurofilament proteins and tau in health and disease.Brain Res Bull. 2023 Jan;192:115-127. doi: 10.1016/j.brainresbull.2022.10.017. Epub 2022 Oct 29. Brain Res Bull. 2023. PMID: 36441047 Free PMC article. Review.
-
RNA Dynamics in Alzheimer's Disease.Molecules. 2021 Aug 24;26(17):5113. doi: 10.3390/molecules26175113. Molecules. 2021. PMID: 34500547 Free PMC article. Review.
-
Truncated Tau caused by intron retention is enriched in Alzheimer's disease cortex and exhibits altered biochemical properties.Proc Natl Acad Sci U S A. 2022 Sep 13;119(37):e2204179119. doi: 10.1073/pnas.2204179119. Epub 2022 Sep 6. Proc Natl Acad Sci U S A. 2022. PMID: 36067305 Free PMC article.
References
-
- Andreadis A, Brown WM, Kosik KS. Structure and novel exons of the human tau gene. Biochemistry. 1992;31:10626–10633. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

