mTOR in Alzheimer disease and its earlier stages: Links to oxidative damage in the progression of this dementing disorder
- PMID: 33933601
- PMCID: PMC8145782
- DOI: 10.1016/j.freeradbiomed.2021.04.025
mTOR in Alzheimer disease and its earlier stages: Links to oxidative damage in the progression of this dementing disorder
Abstract
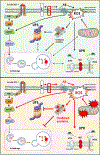
Alzheimer's disease (AD) is the most prevalent form of dementia in the elderly population and has worldwide impact. The etiology of the disease is complex and results from the confluence of multiple mechanisms ultimately leading to neuronal loss and cognitive decline. Among risk factors, aging is the most relevant and accounts for several pathogenic events that contribute to disease-specific toxic mechanisms. Accumulating evidence linked the alterations of the mammalian target of rapamycin (mTOR), a serine/threonine protein kinase playing a key role in the regulation of protein synthesis and degradation, to age-dependent cognitive decline and pathogenesis of AD. To date, growing studies demonstrated that aberrant mTOR signaling in the brain affects several pathways involved in energy metabolism, cell growth, mitochondrial function and proteostasis. Recent advances associated alterations of the mTOR pathway with the increased oxidative stress. Disruption of all these events strongly contribute to age-related cognitive decline including AD. The current review discusses the main regulatory roles of mTOR signaling network in the brain, focusing on its role in autophagy, oxidative stress and energy metabolism. Collectively, experimental data suggest that targeting mTOR in the CNS can be a valuable strategy to prevent/slow the progression of AD.
Keywords: Alzheimer's disease; Oxidative stress; Protein aggregation; Proteostasis; mTOR.
Copyright © 2021 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
The Triangle of Death in Alzheimer's Disease Brain: The Aberrant Cross-Talk Among Energy Metabolism, Mammalian Target of Rapamycin Signaling, and Protein Homeostasis Revealed by Redox Proteomics.Antioxid Redox Signal. 2017 Mar 10;26(8):364-387. doi: 10.1089/ars.2016.6759. Epub 2016 Oct 20. Antioxid Redox Signal. 2017. PMID: 27626216 Review.
-
mTOR signaling in aging and neurodegeneration: At the crossroad between metabolism dysfunction and impairment of autophagy.Neurobiol Dis. 2015 Dec;84:39-49. doi: 10.1016/j.nbd.2015.03.014. Epub 2015 Mar 19. Neurobiol Dis. 2015. PMID: 25796566 Review.
-
mTOR in Down syndrome: Role in Aß and tau neuropathology and transition to Alzheimer disease-like dementia.Free Radic Biol Med. 2018 Jan;114:94-101. doi: 10.1016/j.freeradbiomed.2017.08.009. Epub 2017 Aug 12. Free Radic Biol Med. 2018. PMID: 28807816 Free PMC article. Review.
-
[Roles of Mammalian Target of Rapamycin Signaling and Autophagy Pathway in Alzheimer's Disease].Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2019 Apr 28;41(2):248-255. doi: 10.3881/j.issn.1000-503X.10802. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2019. PMID: 31060682 Review. Chinese.
-
Alteration of mTOR signaling occurs early in the progression of Alzheimer disease (AD): analysis of brain from subjects with pre-clinical AD, amnestic mild cognitive impairment and late-stage AD.J Neurochem. 2015 Jun;133(5):739-49. doi: 10.1111/jnc.13037. Epub 2015 Feb 26. J Neurochem. 2015. PMID: 25645581
Cited by
-
The beneficial role of autophagy in multiple sclerosis: Yes or No?Autophagy. 2024 Feb;20(2):259-274. doi: 10.1080/15548627.2023.2259281. Epub 2023 Sep 15. Autophagy. 2024. PMID: 37712858 Free PMC article. Review.
-
Ubiquitin carboxyl-terminal hydrolase L-1 in brain: Focus on its oxidative/nitrosative modification and role in brains of subjects with Alzheimer disease and mild cognitive impairment.Free Radic Biol Med. 2021 Dec;177:278-286. doi: 10.1016/j.freeradbiomed.2021.10.036. Epub 2021 Nov 1. Free Radic Biol Med. 2021. PMID: 34737037 Free PMC article. Review.
-
Protein restriction slows the development and progression of pathology in a mouse model of Alzheimer's disease.Nat Commun. 2024 Jun 18;15(1):5217. doi: 10.1038/s41467-024-49589-z. Nat Commun. 2024. PMID: 38890307 Free PMC article.
-
The SGLT2 inhibitor empagliflozin exerts neuroprotective effect against hydrogen peroxide-induced toxicity on primary neurons.Metab Brain Dis. 2024 Nov 19;40(1):15. doi: 10.1007/s11011-024-01478-6. Metab Brain Dis. 2024. PMID: 39560812
-
Anti-neoplastic sulfonamides alter the metabolic homeostasis and disrupt the suppressor activity of regulatory T cells.Sci Rep. 2022 Nov 9;12(1):19112. doi: 10.1038/s41598-022-23601-2. Sci Rep. 2022. PMID: 36352020 Free PMC article.
References
-
- McGowan E, Pickford F, Kim J, Onstead L, Eriksen J, Yu C, Skipper L, Murphy MP, Beard J, Das P, Jansen K, DeLucia M, Lin WL, Dolios G, Wang R, Eckman CB, Dickson DW, Hutton M, Hardy J, Golde T, Abeta42 is essential for parenchymal and vascular amyloid deposition in mice, Neuron 47(2) (2005) 191–199. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

