Impaired ATG16L-Dependent Autophagy Promotes Renal Interstitial Fibrosis in Chronic Renal Graft Dysfunction Through Inducing EndMT by NF-κB Signal Pathway
- PMID: 33927720
- PMCID: PMC8076642
- DOI: 10.3389/fimmu.2021.650424
Impaired ATG16L-Dependent Autophagy Promotes Renal Interstitial Fibrosis in Chronic Renal Graft Dysfunction Through Inducing EndMT by NF-κB Signal Pathway
Abstract
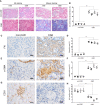
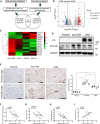
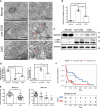
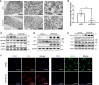
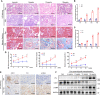
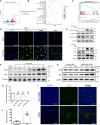
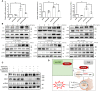
Chronic renal graft dysfunction (CAD) is caused by multiple factors, including glomerular sclerosis, inflammation, interstitial fibrosis and tubular atrophy (IF/TA). However, the most prominent elements of CAD are IF/TA. Our studies have confirmed that endothelial-mesenchymal transition (EndMT) is an important source to allograft IF/TA. The characteristic of EndMT is the loss of endothelial marker and the acquisition of mesenchymal or fibroblastic phenotypes. Autophagy is an intracellular degradation pathway that is regulated by autophagy-related proteins and plays a vital role in many fibrotic conditions. However, whether or not autophagy contributes to fibrosis of renal allograft and how such mechanism occurs still remains unclear. Autophagy related 16 like gene (ATG16L) is a critical autophagy-related gene (ARG) necessary for autophagosome formation. Here, we first analyzed kidney transplant patient tissues from Gene Expression Omnibus (GEO) datasets and 60 transplant patients from our center. Recipients with stable kidney function were defined as non-CAD group and all patients in CAD group were histopathologically diagnosed with CAD. Results showed that ATG16L, as one significant differential ARG, was less expressed in CAD group compared to the non-CAD group. Furthermore, we found there were less autophagosomes and autolysosomes in transplanted kidneys of CAD patients, and downregulation of autophagy is a poor prognostic factor. In vitro, we found out that the knockdown of ATG16L enhanced the process of EndMT in human renal glomerular endothelial cells (HRGECs). In vivo, the changes of EndMT and autophagic flux were then detected in rat renal transplant models of CAD. We demonstrated the occurrence of EndMT, and indicated that abundance of ATG16L was accompanied by the dynamic autophagic flux change along different stages of kidney transplantation. Mechanistically, knockdown of ATG16L, specifically in endothelial cells, reduced of NF-κB degradation and excreted inflammatory cytokines (IL-1β, IL-6 and TNF-α), which could facilitate EndMT. In conclusion, ATG16L-dependent autophagic flux causing by transplant showed progressive loss increase over time. Inflammatory cytokines from this process promoted EndMT, thereby leading to progression of CAD. ATG16L served as a negative regulator of EndMT and development of renal graft fibrosis, and autophagy can be explored as a potential therapeutic target for chronic renal graft dysfunction.
Keywords: ATG16L; EndMT; autophagy; chronic renal graft dysfunction; inflammatory cytokines; renal interstitial fibrosis.
Copyright © 2021 Gui, Suo, Wang, Zheng, Fei, Chen, Sun, Han, Tao, Ju, Yang, Gu and Tan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Everolimus Alleviates Renal Allograft Interstitial Fibrosis by Inhibiting Epithelial-to-Mesenchymal Transition Not Only via Inducing Autophagy but Also via Stabilizing IκB-α.Front Immunol. 2022 Jan 24;12:753412. doi: 10.3389/fimmu.2021.753412. eCollection 2021. Front Immunol. 2022. PMID: 35140705 Free PMC article.
-
Curcumin Blunts IL-6 Dependent Endothelial-to-Mesenchymal Transition to Alleviate Renal Allograft Fibrosis Through Autophagy Activation.Front Immunol. 2021 May 28;12:656242. doi: 10.3389/fimmu.2021.656242. eCollection 2021. Front Immunol. 2021. Retraction in: Front Immunol. 2021 Nov 26;12:818539. doi: 10.3389/fimmu.2021.818539. PMID: 34122411 Free PMC article. Retracted.
-
Rictor/mTORC2 signalling contributes to renal vascular endothelial-to-mesenchymal transition and renal allograft interstitial fibrosis by regulating BNIP3-mediated mitophagy.Clin Transl Med. 2024 May;14(5):e1686. doi: 10.1002/ctm2.1686. Clin Transl Med. 2024. PMID: 38769658 Free PMC article.
-
Endothelial-to-mesenchymal transition: Cytokine-mediated pathways that determine endothelial fibrosis under inflammatory conditions.Cytokine Growth Factor Rev. 2017 Feb;33:41-54. doi: 10.1016/j.cytogfr.2016.09.002. Epub 2016 Sep 23. Cytokine Growth Factor Rev. 2017. PMID: 27692608 Review.
-
Role of Endothelial Cells in Renal Fibrosis.Adv Exp Med Biol. 2019;1165:145-163. doi: 10.1007/978-981-13-8871-2_8. Adv Exp Med Biol. 2019. PMID: 31399965 Review.
Cited by
-
Emerging roles of inflammation-mediated endothelial-mesenchymal transition in health and disease.Inflamm Regen. 2022 Feb 7;42(1):9. doi: 10.1186/s41232-021-00186-3. Inflamm Regen. 2022. PMID: 35130955 Free PMC article. Review.
-
Plasma-derived exosomes contributes to endothelial-to-mesenchymal transition in Moyamoya disease.Heliyon. 2024 Feb 22;10(5):e26748. doi: 10.1016/j.heliyon.2024.e26748. eCollection 2024 Mar 15. Heliyon. 2024. PMID: 38434376 Free PMC article.
-
Cell death induced by acute renal injury: a perspective on the contributions of accidental and programmed cell death.Am J Physiol Renal Physiol. 2024 Jul 1;327(1):F4-F20. doi: 10.1152/ajprenal.00275.2023. Epub 2024 Apr 25. Am J Physiol Renal Physiol. 2024. PMID: 38660714 Review.
-
Src inhibition modulates AMBRA1-mediated mitophagy to counteract endothelial-to-mesenchymal transition in renal allograft fibrosis.Cell Prolif. 2024 Nov;57(11):e13699. doi: 10.1111/cpr.13699. Epub 2024 Jun 29. Cell Prolif. 2024. PMID: 38943534 Free PMC article.
-
Autophagy and Renal Fibrosis.Aging Dis. 2022 Jun 1;13(3):712-731. doi: 10.14336/AD.2021.1027. eCollection 2022 Jun. Aging Dis. 2022. PMID: 35656109 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

