Elevated M-MDSCs in Circulation Are Indicative of Poor Prognosis in Diffuse Large B-Cell Lymphoma Patients
- PMID: 33921711
- PMCID: PMC8074013
- DOI: 10.3390/jcm10081768
Elevated M-MDSCs in Circulation Are Indicative of Poor Prognosis in Diffuse Large B-Cell Lymphoma Patients
Abstract
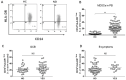
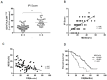
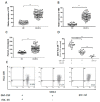
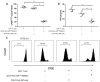
Myeloid-derived suppressor cells (MDSCs) are defined as negative regulators that suppress the immune response through a variety of mechanisms, which usually cluster in cancer, inflammation, and autoimmune diseases. This study aims to investigate the correlation between M-MDSCs and the clinical features of diffuse large B-cell lymphoma (DLBCL) patients, as well as the possible accumulation mechanism of M-MDSCs. The level of M-MDSCs is significantly increased in newly diagnosed and relapsed DLBCL patients. Regarding newly diagnosed DLBCL patients, the frequency of M-MDSCs is positively correlated with tumor progression and negatively correlated with overall survival (OS). More importantly, the level of M-MDSCs can be defined as a biomarker for a poor prognosis in DLBCL patients. Additionally, interleukin-35 (IL-35) mediates the accumulation of M-MDSCs in DLBCL patients. Anti-IL-35 treatment significantly reduces levels of M-MDSCs in Ly8 tumor-bearing mice. Thus, M-MDSCs are involved in the pathological process of DLBCL. Targeting M-MDSCs may be a promising therapeutic strategy for the treatment of DLBCL patients.
Keywords: diffuse large B-cell lymphoma; immunosuppression; interleukin-35; myeloid-derived suppressor cells; prognosis; tumor progression.
Conflict of interest statement
All authors declare no conflict of interest.
Figures


Similar articles
-
Surface TREM2 on circulating M-MDSCs as a novel prognostic factor for adults with treatment-naïve diffuse large B-cell lymphoma.Exp Hematol Oncol. 2023 Apr 7;12(1):35. doi: 10.1186/s40164-023-00399-x. Exp Hematol Oncol. 2023. PMID: 37029450 Free PMC article.
-
Elevated circulating myeloid-derived suppressor cells associated with poor prognosis in B-cell non-Hodgkin's lymphoma patients.Immun Inflamm Dis. 2022 May;10(5):e616. doi: 10.1002/iid3.616. Immun Inflamm Dis. 2022. PMID: 35478441 Free PMC article.
-
Absolute monocytosis at diagnosis correlates with survival in diffuse large B-cell lymphoma-possible link with monocytic myeloid-derived suppressor cells.Hematol Oncol. 2013 Jun;31(2):65-71. doi: 10.1002/hon.2019. Epub 2012 Jun 20. Hematol Oncol. 2013. PMID: 22714941
-
Unraveling the Immune Microenvironment in Diffuse Large B-Cell Lymphoma: Prognostic and Potential Therapeutic Implications.Curr Issues Mol Biol. 2024 Jul 5;46(7):7048-7064. doi: 10.3390/cimb46070420. Curr Issues Mol Biol. 2024. PMID: 39057061 Free PMC article. Review.
-
Suppression of T cells by myeloid-derived suppressor cells in cancer.Hum Immunol. 2017 Feb;78(2):113-119. doi: 10.1016/j.humimm.2016.12.001. Epub 2016 Dec 7. Hum Immunol. 2017. PMID: 27939507 Review.
Cited by
-
Interleukin-35: Structure, Function and Its Impact on Immune-Related Diseases.J Interferon Cytokine Res. 2021 Nov;41(11):391-406. doi: 10.1089/jir.2021.0147. J Interferon Cytokine Res. 2021. PMID: 34788131 Free PMC article. Review.
-
Key Players of the Immunosuppressive Tumor Microenvironment and Emerging Therapeutic Strategies.Front Cell Dev Biol. 2022 Mar 8;10:830208. doi: 10.3389/fcell.2022.830208. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35345849 Free PMC article. Review.
-
Epigenetic, Metabolic, and Immune Crosstalk in Germinal-Center-Derived B-Cell Lymphomas: Unveiling New Vulnerabilities for Rational Combination Therapies.Front Cell Dev Biol. 2022 Jan 7;9:805195. doi: 10.3389/fcell.2021.805195. eCollection 2021. Front Cell Dev Biol. 2022. PMID: 35071240 Free PMC article. Review.
-
Myeloid-derived suppressor cells: key immunosuppressive regulators and therapeutic targets in hematological malignancies.Biomark Res. 2023 Mar 29;11(1):34. doi: 10.1186/s40364-023-00475-8. Biomark Res. 2023. PMID: 36978204 Free PMC article. Review.
-
Suppressing MDSC Recruitment to the Tumor Microenvironment by Antagonizing CXCR2 to Enhance the Efficacy of Immunotherapy.Cancers (Basel). 2021 Dec 15;13(24):6293. doi: 10.3390/cancers13246293. Cancers (Basel). 2021. PMID: 34944914 Free PMC article. Review.
References
-
- Tomita N., Yokoyama M., Yamamoto W., Watanabe R., Shimazu Y., Masaki Y., Tsunoda S., Hashimoto C., Murayama K., Yano T., et al. The standard international prognostic index for predicting the risk of CNS involvement in DLBCL without specific prophylaxis. Leuk. Lymphoma. 2017;59:97–104. doi: 10.1080/10428194.2017.1330541. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

