Signaling Pathways in Bone Development and Their Related Skeletal Dysplasia
- PMID: 33919228
- PMCID: PMC8122623
- DOI: 10.3390/ijms22094321
Signaling Pathways in Bone Development and Their Related Skeletal Dysplasia
Abstract
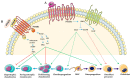
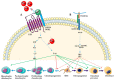
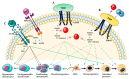
Bone development is a tightly regulated process. Several integrated signaling pathways including HH, PTHrP, WNT, NOTCH, TGF-β, BMP, FGF and the transcription factors SOX9, RUNX2 and OSX are essential for proper skeletal development. Misregulation of these signaling pathways can cause a large spectrum of congenital conditions categorized as skeletal dysplasia. Since the signaling pathways involved in skeletal dysplasia interact at multiple levels and have a different role depending on the time of action (early or late in chondrogenesis and osteoblastogenesis), it is still difficult to precisely explain the physiopathological mechanisms of skeletal disorders. However, in recent years, significant progress has been made in elucidating the mechanisms of these signaling pathways and genotype-phenotype correlations have helped to elucidate their role in skeletogenesis. Here, we review the principal signaling pathways involved in bone development and their associated skeletal dysplasia.
Keywords: bone development; signaling pathways; skeletal dysplasia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
TGF-β and BMP Signaling Pathways in Skeletal Dysplasia with Short and Tall Stature.Annu Rev Genomics Hum Genet. 2023 Aug 25;24:225-253. doi: 10.1146/annurev-genom-120922-094107. Annu Rev Genomics Hum Genet. 2023. PMID: 37624666 Review.
-
Signaling Pathways in Craniofacial Development: Insights from Rare Skeletal Disorders.Curr Top Dev Biol. 2015;115:493-542. doi: 10.1016/bs.ctdb.2015.09.005. Epub 2015 Oct 23. Curr Top Dev Biol. 2015. PMID: 26589936 Review.
-
A combined series of Fgf9 and Fgf18 mutant alleles identifies unique and redundant roles in skeletal development.Dev Biol. 2016 Mar 1;411(1):72-84. doi: 10.1016/j.ydbio.2016.01.008. Epub 2016 Jan 16. Dev Biol. 2016. PMID: 26794256 Free PMC article.
-
Multiple mechanisms are involved in inhibition of osteoblast differentiation by PTHrP and PTH in KS483 Cells.J Bone Miner Res. 2005 Dec;20(12):2233-44. doi: 10.1359/JBMR.050821. Epub 2005 Aug 29. J Bone Miner Res. 2005. PMID: 16294276
-
FGF and FGFR signaling in chondrodysplasias and craniosynostosis.J Cell Biochem. 2005 Dec 1;96(5):888-96. doi: 10.1002/jcb.20582. J Cell Biochem. 2005. PMID: 16149058 Review.
Cited by
-
Conditioned Medium of Intervertebral Disc Cells Inhibits Osteo-Genesis on Autologous Bone-Marrow-Derived Mesenchymal Stromal Cells and Osteoblasts.Biomedicines. 2024 Feb 6;12(2):376. doi: 10.3390/biomedicines12020376. Biomedicines. 2024. PMID: 38397978 Free PMC article.
-
Hedgehog-Related Mutation Causes Bone Malformations with or without Hereditary Gene Mutations.Int J Mol Sci. 2023 Aug 17;24(16):12903. doi: 10.3390/ijms241612903. Int J Mol Sci. 2023. PMID: 37629084 Free PMC article. Review.
-
Differential gene expression in the calvarial and cortical bone of juvenile female mice.Front Endocrinol (Lausanne). 2023 Jun 12;14:1127536. doi: 10.3389/fendo.2023.1127536. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37378024 Free PMC article.
-
TGF-β signaling regulates differentiation of MSCs in bone metabolism: disputes among viewpoints.Stem Cell Res Ther. 2024 May 31;15(1):156. doi: 10.1186/s13287-024-03761-w. Stem Cell Res Ther. 2024. PMID: 38816830 Free PMC article. Review.
-
Changes in skeletal dysplasia nosology.Rom J Morphol Embryol. 2021 Jul-Sep;62(3):689-696. doi: 10.47162/RJME.62.3.05. Rom J Morphol Embryol. 2021. PMID: 35263396 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

