Castration causes an increase in lysosomal size and upregulation of cathepsin D expression in principal cells along with increased secretion of procathepsin D and prosaposin oligomers in adult rat epididymis
- PMID: 33914781
- PMCID: PMC8084160
- DOI: 10.1371/journal.pone.0250454
Castration causes an increase in lysosomal size and upregulation of cathepsin D expression in principal cells along with increased secretion of procathepsin D and prosaposin oligomers in adult rat epididymis
Abstract
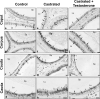
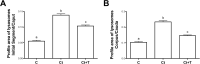
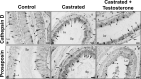
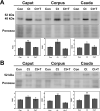
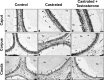
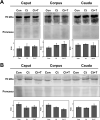
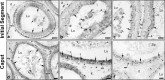
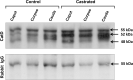
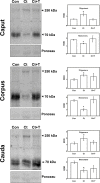
In the epididymis, lysosomal proteins of the epithelial cells are normally targeted from the Golgi apparatus to lysosomes for degradation, although their secretion into the epididymal lumen has been documented and associated with sperm maturation. In this study, cathepsin D (CatD) and prosaposin (PSAP) were examined in adult epididymis of control, and 2-day castrated rats without (Ct) and with testosterone replacement (Ct+T) to evaluate their expression and regulation within epididymal epithelial cells. By light microscope-immunocytochemistry, a quantitative increase in size of lysosomes in principal cells of Ct animals was noted from the distal initial segment to the proximal cauda. Androgen replacement did not restore the size of lysosomes to control levels. Western blot analysis revealed a significant increase in CatD expression in the epididymis of Ct animals, which suggested an upregulation of its expression in principal cells; androgens restored levels of CatD to that of controls. In contrast, PSAP expression in Ct animals was not altered from controls. Additionally, an increase in procathepsin D levels was noted from samples of the epididymal fluid of Ct compared to control animals, accompanied by an increased complex formation with PSAP. Moreover, an increased oligomerization of prosaposin was observed in the epididymal lumen of Ct rats, with changes reverted to controls in Ct+T animals. Taken together these data suggest castration causes an increased uptake of substrates that are acted upon by CatD in lysosomes of principal cells and in the lumen by procathepsin D. These substrates may be derived from apoptotic cells noted in the lumen of proximal regions and possibly by degenerating sperm in distal regions of the epididymis of Ct animals. Exploring the mechanisms by which lysosomal enzymes are synthesized and secreted by the epididymis may help resolve some of the issues originating from epididymal dysfunctions with relevance to sperm maturation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Sortilin knock-down alters the expression and distribution of cathepsin D and prosaposin and up-regulates the cation-dependent mannose-6-phosphate receptor in rat epididymal cells.Sci Rep. 2023 Mar 1;13(1):3461. doi: 10.1038/s41598-023-29157-z. Sci Rep. 2023. PMID: 36859404 Free PMC article.
-
Regulation of sulfated glycoprotein-1 and cathepsin D expression in adult rat epididymis.J Androl. 2003 May-Jun;24(3):408-22. doi: 10.1002/j.1939-4640.2003.tb02690.x. J Androl. 2003. PMID: 12721218
-
Cell- and region-specific localization of lysosomal and secretory proteins and endocytic receptors in epithelial cells of the cauda epididymidis and vas deferens of the adult rat.J Androl. 1999 May-Jun;20(3):415-29. J Androl. 1999. PMID: 10386822
-
Pro-cathepsin D, Prosaposin, and Progranulin: Lysosomal Networks in Parkinsonism.Trends Mol Med. 2020 Oct;26(10):913-923. doi: 10.1016/j.molmed.2020.07.004. Epub 2020 Sep 15. Trends Mol Med. 2020. PMID: 32948448 Free PMC article. Review.
-
Epididymal functions and their hormonal regulation.Aust J Biol Sci. 1983;36(3):205-21. doi: 10.1071/bi9830205. Aust J Biol Sci. 1983. PMID: 6360113 Review.
Cited by
-
Distinct actions of testicular endocrine and lumicrine signaling on the proximal epididymal transcriptome.Reprod Biol Endocrinol. 2024 Apr 10;22(1):40. doi: 10.1186/s12958-024-01213-x. Reprod Biol Endocrinol. 2024. PMID: 38600586 Free PMC article.
-
Busulfan administration replicated the characteristics of the epididymal initial segment observed in mice lacking testis-epididymis lumicrine signaling.J Reprod Dev. 2024 Apr 4;70(2):104-114. doi: 10.1262/jrd.2023-102. Epub 2024 Feb 9. J Reprod Dev. 2024. PMID: 38346723 Free PMC article.
-
Identification of postnatal development dependent genes and proteins in porcine epididymis.BMC Genomics. 2023 Dec 4;24(1):729. doi: 10.1186/s12864-023-09827-y. BMC Genomics. 2023. PMID: 38049726 Free PMC article.
-
Effects of Heparan sulfate acetyl-CoA: Alpha-glucosaminide N-acetyltransferase (HGSNAT) inactivation on the structure and function of epithelial and immune cells of the testis and epididymis and sperm parameters in adult mice.PLoS One. 2023 Sep 27;18(9):e0292157. doi: 10.1371/journal.pone.0292157. eCollection 2023. PLoS One. 2023. PMID: 37756356 Free PMC article.
-
Sortilin knock-down alters the expression and distribution of cathepsin D and prosaposin and up-regulates the cation-dependent mannose-6-phosphate receptor in rat epididymal cells.Sci Rep. 2023 Mar 1;13(1):3461. doi: 10.1038/s41598-023-29157-z. Sci Rep. 2023. PMID: 36859404 Free PMC article.
References
-
- Robaire B, Hermo L. Efferent Ducts, epididymis and vas deferens: structure, functions, and their regulation. In: Knobil E, Neill J, editors. The Physiology of Reproduction. New York: Raven Press, Ltd.; 1988. pp. 999–1080.
-
- Hamilton DW. Structure and function of the epithelium lining efferentes, ductus epididymidis and ductus deferens in the rat. In: Hamilton D, Greep R, editors. Handbook of Physiology. Washington DC: American Physiological Society; 1975. pp. 259–301. Available: https://ci.nii.ac.jp/naid/10025550105
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

