VPS13D bridges the ER to mitochondria and peroxisomes via Miro
- PMID: 33891013
- PMCID: PMC8077184
- DOI: 10.1083/jcb.202010004
VPS13D bridges the ER to mitochondria and peroxisomes via Miro
Erratum in
-
Correction: VPS13D bridges the ER to mitochondria and peroxisomes via Miro.J Cell Biol. 2021 Aug 2;220(8):e20201000405052021c. doi: 10.1083/jcb.20201000405052021c. Epub 2021 Jun 22. J Cell Biol. 2021. PMID: 34156432 Free PMC article. No abstract available.
Abstract
Mitochondria, which are excluded from the secretory pathway, depend on lipid transport proteins for their lipid supply from the ER, where most lipids are synthesized. In yeast, the outer mitochondrial membrane GTPase Gem1 is an accessory factor of ERMES, an ER-mitochondria tethering complex that contains lipid transport domains and that functions, partially redundantly with Vps13, in lipid transfer between the two organelles. In metazoa, where VPS13, but not ERMES, is present, the Gem1 orthologue Miro was linked to mitochondrial dynamics but not to lipid transport. Here we show that Miro, including its peroxisome-enriched splice variant, recruits the lipid transport protein VPS13D, which in turn binds the ER in a VAP-dependent way and thus could provide a lipid conduit between the ER and mitochondria. These findings reveal a so far missing link between function(s) of Gem1/Miro in yeast and higher eukaryotes, where Miro is a Parkin substrate, with potential implications for Parkinson's disease pathogenesis.
© 2021 Guillén-Samander et al.
Figures



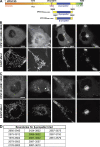
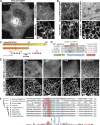
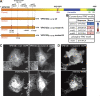
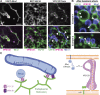
Similar articles
-
The conserved GTPase Gem1 regulates endoplasmic reticulum-mitochondria connections.Proc Natl Acad Sci U S A. 2011 Aug 23;108(34):14151-6. doi: 10.1073/pnas.1111314108. Epub 2011 Aug 8. Proc Natl Acad Sci U S A. 2011. PMID: 21825164 Free PMC article.
-
The ERMES complex and ER-mitochondria connections.Biochem Soc Trans. 2012 Apr;40(2):445-50. doi: 10.1042/BST20110758. Biochem Soc Trans. 2012. PMID: 22435828 Review.
-
VPS13D interacts with VCP/p97 and negatively regulates endoplasmic reticulum-mitochondria interactions.Mol Biol Cell. 2021 Aug 1;32(16):1474-1486. doi: 10.1091/mbc.E21-03-0097. Epub 2021 Jun 16. Mol Biol Cell. 2021. PMID: 34133214 Free PMC article.
-
Gem1 and ERMES do not directly affect phosphatidylserine transport from ER to mitochondria or mitochondrial inheritance.Traffic. 2012 Jun;13(6):880-90. doi: 10.1111/j.1600-0854.2012.01352.x. Epub 2012 Apr 8. Traffic. 2012. PMID: 22409400 Free PMC article.
-
Mitochondrial Miro GTPases coordinate mitochondrial and peroxisomal dynamics.Small GTPases. 2021 Sep-Nov;12(5-6):372-398. doi: 10.1080/21541248.2020.1843957. Epub 2020 Nov 12. Small GTPases. 2021. PMID: 33183150 Free PMC article. Review.
Cited by
-
Sharing the wealth: The versatility of proteins targeted to peroxisomes and other organelles.Front Cell Dev Biol. 2022 Sep 26;10:934331. doi: 10.3389/fcell.2022.934331. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36225313 Free PMC article. Review.
-
A Possible Role of VPS13B in the Formation of Golgi-Lipid Droplet Contacts Associating with the ER.Contact (Thousand Oaks). 2023 Sep 11;6:25152564231195718. doi: 10.1177/25152564231195718. eCollection 2023 Jan-Dec. Contact (Thousand Oaks). 2023. PMID: 38090145 Free PMC article.
-
SHIP164 is a chorein motif lipid transfer protein that controls endosome-Golgi membrane traffic.J Cell Biol. 2022 Jun 6;221(6):e202111018. doi: 10.1083/jcb.202111018. Epub 2022 May 2. J Cell Biol. 2022. PMID: 35499567 Free PMC article.
-
Biogenesis of Rab14-positive endosome buds at Golgi-endosome contacts by the RhoBTB3-SHIP164-Vps26B complex.Cell Discov. 2024 Apr 2;10(1):38. doi: 10.1038/s41421-024-00651-6. Cell Discov. 2024. PMID: 38565878 Free PMC article.
-
Proteomic profiling of the oncogenic septin 9 reveals isoform-specific interactions in breast cancer cells.Proteomics. 2021 Oct;21(19):e2100155. doi: 10.1002/pmic.202100155. Epub 2021 Aug 31. Proteomics. 2021. PMID: 34409731 Free PMC article.
References
-
- Akşit, A. 2018. Peroxisomal Membrane Contact Sites in the Yeast Hansenula Polymorpha. PhD Thesis. University of Groningen, Groningen, Netherlands. Available at: https://research.rug.nl/en/publications/peroxisomal-membrane-contact-sit....
-
- Anding, A.L., Wang C., Chang T.-K., Sliter D.A., Powers C.M., Hofmann K., Youle R.J., and Baehrecke E.H.. 2018. Vps13D encodes a ubiquitin-binding protein that is required for the regulation of mitochondrial size and clearance. Curr. Biol. 28:287–295.e6. 10.1016/j.cub.2017.11.064 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

