Mesenchymal stem/stromal cell-based therapy: mechanism, systemic safety and biodistribution for precision clinical applications
- PMID: 33849537
- PMCID: PMC8043779
- DOI: 10.1186/s12929-021-00725-7
Mesenchymal stem/stromal cell-based therapy: mechanism, systemic safety and biodistribution for precision clinical applications
Abstract
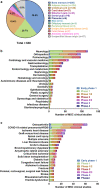
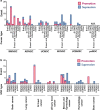
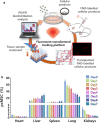
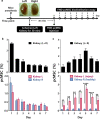
Mesenchymal stem/stromal cells (MSCs) are a promising resource for cell-based therapy because of their high immunomodulation ability, tropism towards inflamed and injured tissues, and their easy access and isolation. Currently, there are more than 1200 registered MSC clinical trials globally. However, a lack of standardized methods to characterize cell safety, efficacy, and biodistribution dramatically hinders the progress of MSC utility in clinical practice. In this review, we summarize the current state of MSC-based cell therapy, focusing on the systemic safety and biodistribution of MSCs. MSC-associated risks of tumor initiation and promotion and the underlying mechanisms of these risks are discussed. In addition, MSC biodistribution methodology and the pharmacokinetics and pharmacodynamics of cell therapies are addressed. Better understanding of the systemic safety and biodistribution of MSCs will facilitate future clinical applications of precision medicine using stem cells.
Keywords: Cell therapy; Mesenchymal stem/stromal cell; Single cell imaging; Systemic safety; biodistribution.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Mesenchymal stromal cell therapies: immunomodulatory properties and clinical progress.Stem Cell Res Ther. 2020 Aug 8;11(1):345. doi: 10.1186/s13287-020-01855-9. Stem Cell Res Ther. 2020. PMID: 32771052 Free PMC article. Review.
-
In vivo safety profile and biodistribution of GMP-manufactured human skin-derived ABCB5-positive mesenchymal stromal cells for use in clinical trials.Cytotherapy. 2019 May;21(5):546-560. doi: 10.1016/j.jcyt.2018.12.005. Epub 2019 Mar 14. Cytotherapy. 2019. PMID: 30878384 Free PMC article.
-
Mesenchymal stem/stromal cells in cancer therapy.J Hematol Oncol. 2021 Nov 17;14(1):195. doi: 10.1186/s13045-021-01208-w. J Hematol Oncol. 2021. PMID: 34789315 Free PMC article. Review.
-
Safety Profile of Good Manufacturing Practice Manufactured Interferon γ-Primed Mesenchymal Stem/Stromal Cells for Clinical Trials.Stem Cells Transl Med. 2017 Oct;6(10):1868-1879. doi: 10.1002/sctm.16-0485. Epub 2017 Sep 9. Stem Cells Transl Med. 2017. PMID: 28887912 Free PMC article.
-
Mesenchymal Stem/Stromal Cell-Based Therapies in Systemic Rheumatic Disease: From Challenges to New Approaches for Overcoming Restrictions.Int J Mol Sci. 2023 Jun 15;24(12):10161. doi: 10.3390/ijms241210161. Int J Mol Sci. 2023. PMID: 37373308 Free PMC article. Review.
Cited by
-
Repair of spinal cord injury by bone marrow mesenchymal stem cell-derived exosomes: a systematic review and meta-analysis based on rat models.Front Mol Neurosci. 2024 Aug 7;17:1448777. doi: 10.3389/fnmol.2024.1448777. eCollection 2024. Front Mol Neurosci. 2024. PMID: 39169950 Free PMC article.
-
Non-clinical assessment of safety, biodistribution and tumorigenicity of human mesenchymal stromal cells.Toxicol Rep. 2021 Nov 29;8:1960-1969. doi: 10.1016/j.toxrep.2021.11.016. eCollection 2021. Toxicol Rep. 2021. PMID: 34926173 Free PMC article.
-
From Primary MSC Culture of Adipose Tissue to Immortalized Cell Line Producing Cytokines for Potential Use in Regenerative Medicine Therapy or Immunotherapy.Int J Mol Sci. 2021 Oct 23;22(21):11439. doi: 10.3390/ijms222111439. Int J Mol Sci. 2021. PMID: 34768869 Free PMC article.
-
Novel Potency Assay for MSC Secretome-Based Treatment of Idiopathic Male Infertility Employed Leydig Cells and Revealed Vascular Endothelial Growth Factor as a Promising Potency Marker.Int J Mol Sci. 2022 Aug 20;23(16):9414. doi: 10.3390/ijms23169414. Int J Mol Sci. 2022. PMID: 36012677 Free PMC article.
-
A1-reprogrammed mesenchymal stromal cells prime potent antitumoral responses.iScience. 2024 Feb 17;27(3):109248. doi: 10.1016/j.isci.2024.109248. eCollection 2024 Mar 15. iScience. 2024. PMID: 38433914 Free PMC article.
References
-
- Friedenstein AJ, Piatetzky S, II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16(3):381–390. - PubMed
Publication types
MeSH terms
Grants and funding
- MOST 107-2321-B-038-002, MOST 107-2314-B-038-061, MOST108-2314-B-038-006, MOST108-2321-B-038-003, MOST109-2314-B-038-135, MOST109-2321-B-038-003/Ministry of Science and Technology, Taiwan
- MOST105-2325-B-002-040, MOST106-3114-B-038-001, MOST107-2321-B-038 -002, MOST108-2321-B-038-003/Ministry of Science and Technology, Taiwan
LinkOut - more resources
Full Text Sources
Other Literature Sources

