Exosomes derived from miR-26a-modified MSCs promote axonal regeneration via the PTEN/AKT/mTOR pathway following spinal cord injury
- PMID: 33820561
- PMCID: PMC8022427
- DOI: 10.1186/s13287-021-02282-0
Exosomes derived from miR-26a-modified MSCs promote axonal regeneration via the PTEN/AKT/mTOR pathway following spinal cord injury
Abstract
Background: Exosomes derived from the bone marrow mesenchymal stem cell (MSC) have shown great potential in spinal cord injury (SCI) treatment. This research was designed to investigate the therapeutic effects of miR-26a-modified MSC-derived exosomes (Exos-26a) following SCI.
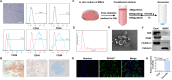
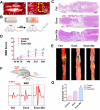
Methods: Bioinformatics and data mining were performed to explore the role of miR-26a in SCI. Exosomes were isolated from miR-26a-modified MSC culture medium by ultracentrifugation. A series of experiments, including assessment of Basso, Beattie and Bresnahan scale, histological evaluation, motor-evoked potential recording, diffusion tensor imaging, and western blotting, were performed to determine the therapeutic influence and the underlying molecular mechanisms of Exos-26a in SCI rats.
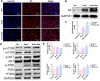
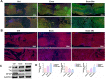
Results: Exos-26a was shown to promote axonal regeneration. Furthermore, we found that exosomes derived from miR-26a-modified MSC could improve neurogenesis and attenuate glial scarring through PTEN/AKT/mTOR signaling cascades.
Conclusions: Exosomes derived from miR-26a-modified MSC could activate the PTEN-AKT-mTOR pathway to promote axonal regeneration and neurogenesis and attenuate glia scarring in SCI and thus present great potential for SCI treatment.
Keywords: Axonal regeneration; Exosomes; Mesenchymal stem cells; Spinal cord injury; miR-26a/PTEN axis.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
BMSC-Derived Exosomes Carrying miR-26a-5p Ameliorate Spinal Cord Injury via Negatively Regulating EZH2 and Activating the BDNF-TrkB-CREB Signaling.Mol Neurobiol. 2024 Oct;61(10):8156-8174. doi: 10.1007/s12035-024-04082-y. Epub 2024 Mar 13. Mol Neurobiol. 2024. PMID: 38478142
-
MiR-212-3p improves rat functional recovery and inhibits neurocyte apoptosis in spinal cord injury models via PTEN downregulation-mediated activation of AKT/mTOR pathway.Brain Res. 2021 Oct 1;1768:147576. doi: 10.1016/j.brainres.2021.147576. Epub 2021 Jul 1. Brain Res. 2021. PMID: 34216580
-
Gypenoside XVII protects against spinal cord injury in mice by regulating the microRNA‑21‑mediated PTEN/AKT/mTOR pathway.Int J Mol Med. 2021 Aug;48(2):146. doi: 10.3892/ijmm.2021.4979. Epub 2021 Jun 16. Int J Mol Med. 2021. PMID: 34132355 Free PMC article.
-
Mesenchymal stem cell-derived exosomes: therapeutic opportunities and challenges for spinal cord injury.Stem Cell Res Ther. 2021 Feb 3;12(1):102. doi: 10.1186/s13287-021-02153-8. Stem Cell Res Ther. 2021. PMID: 33536064 Free PMC article. Review.
-
Exosomes Derived from Mesenchymal Stem Cells: Therapeutic Opportunities for Spinal Cord Injury.Bull Exp Biol Med. 2024 Apr;176(6):716-721. doi: 10.1007/s10517-024-06095-y. Epub 2024 Jun 18. Bull Exp Biol Med. 2024. PMID: 38888648 Review.
Cited by
-
Therapeutic Effect of Exosomes Derived From Stem Cells in Spinal Cord Injury: A Systematic Review Based on Animal Studies.Front Neurol. 2022 Mar 10;13:847444. doi: 10.3389/fneur.2022.847444. eCollection 2022. Front Neurol. 2022. PMID: 35356459 Free PMC article.
-
Exosome-mediated repair of spinal cord injury: a promising therapeutic strategy.Stem Cell Res Ther. 2024 Jan 2;15(1):6. doi: 10.1186/s13287-023-03614-y. Stem Cell Res Ther. 2024. PMID: 38167108 Free PMC article. Review.
-
A swift expanding trend of extracellular vesicles in spinal cord injury research: a bibliometric analysis.J Nanobiotechnology. 2023 Aug 23;21(1):289. doi: 10.1186/s12951-023-02051-6. J Nanobiotechnology. 2023. PMID: 37612689 Free PMC article. Review.
-
Extracellular vesicles as nanotheranostic platforms for targeted neurological disorder interventions.Nano Converg. 2024 May 13;11(1):19. doi: 10.1186/s40580-024-00426-5. Nano Converg. 2024. PMID: 38739358 Free PMC article. Review.
-
mTOR signalling pathway in stem cell bioactivities and angiogenesis potential.Cell Prolif. 2023 Dec;56(12):e13499. doi: 10.1111/cpr.13499. Epub 2023 May 8. Cell Prolif. 2023. PMID: 37156724 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

