SLC6A14 and SLC38A5 Drive the Glutaminolysis and Serine-Glycine-One-Carbon Pathways in Cancer
- PMID: 33806675
- PMCID: PMC8000594
- DOI: 10.3390/ph14030216
SLC6A14 and SLC38A5 Drive the Glutaminolysis and Serine-Glycine-One-Carbon Pathways in Cancer
Abstract
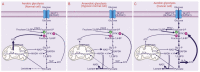
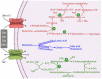
The glutaminolysis and serine-glycine-one-carbon pathways represent metabolic reactions that are reprogramed and upregulated in cancer; these pathways are involved in supporting the growth and proliferation of cancer cells. Glutaminolysis participates in the production of lactate, an oncometabolite, and also in anabolic reactions leading to the synthesis of fatty acids and cholesterol. The serine-glycine-one-carbon pathway is involved in the synthesis of purines and pyrimidines and the control of the epigenetic signature (DNA methylation, histone methylation) in cancer cells. Methionine is obligatory for most of the methyl-transfer reactions in the form of S-adenosylmethionine; here, too, the serine-glycine-one-carbon pathway is necessary for the resynthesis of methionine following the methyl-transfer reaction. Glutamine, serine, glycine, and methionine are obligatory to fuel these metabolic pathways. The first three amino acids can be synthesized endogenously to some extent, but the need for these amino acids in cancer cells is so high that they also have to be acquired from extracellular sources. Methionine is an essential amino acid, thus making it necessary for cancer cells to acquire this amino acid solely from the extracellular milieu. Cancer cells upregulate specific amino acid transporters to meet this increased demand for these four amino acids. SLC6A14 and SLC38A5 are the two transporters that are upregulated in a variety of cancers to mediate the influx of glutamine, serine, glycine, and methionine into cancer cells. SLC6A14 is a Na+/Cl- -coupled transporter for multiple amino acids, including these four amino acids. In contrast, SLC38A5 is a Na+-coupled transporter with rather restricted specificity towards glutamine, serine, glycine, and methionine. Both transporters exhibit unique functional features that are ideal for the rapid proliferation of cancer cells. As such, these two amino acid transporters play a critical role in promoting the survival and growth of cancer cells and hence represent novel, hitherto largely unexplored, targets for cancer therapy.
Keywords: SLC6A14 and SLC38A5; amino acid transporters; cancer-specific metabolism; glutamine addiction; oncometabolites; one-carbon metabolism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Expression and function of SLC38A5, an amino acid-coupled Na+/H+ exchanger, in triple-negative breast cancer and its relevance to macropinocytosis.Biochem J. 2021 Nov 12;478(21):3957-3976. doi: 10.1042/BCJ20210585. Biochem J. 2021. PMID: 34704597 Free PMC article.
-
The SLC38A5/SNAT5 amino acid transporter: from pathophysiology to pro-cancer roles in the tumor microenvironment.Am J Physiol Cell Physiol. 2023 Aug 1;325(2):C550-C562. doi: 10.1152/ajpcell.00169.2023. Epub 2023 Jul 17. Am J Physiol Cell Physiol. 2023. PMID: 37458433 Review.
-
Metabolic Signature of Warburg Effect in Cancer: An Effective and Obligatory Interplay between Nutrient Transporters and Catabolic/Anabolic Pathways to Promote Tumor Growth.Cancers (Basel). 2024 Jan 24;16(3):504. doi: 10.3390/cancers16030504. Cancers (Basel). 2024. PMID: 38339256 Free PMC article. Review.
-
Induction of Oxidative Stress and Ferroptosis in Triple-Negative Breast Cancer Cells by Niclosamide via Blockade of the Function and Expression of SLC38A5 and SLC7A11.Antioxidants (Basel). 2024 Feb 27;13(3):291. doi: 10.3390/antiox13030291. Antioxidants (Basel). 2024. PMID: 38539825 Free PMC article.
-
Analysis of glucose-derived amino acids involved in one-carbon and cancer metabolism by stable-isotope tracing gas chromatography mass spectrometry.Anal Biochem. 2019 Feb 1;566:1-9. doi: 10.1016/j.ab.2018.10.026. Epub 2018 Oct 26. Anal Biochem. 2019. PMID: 30409761 Free PMC article.
Cited by
-
ZNF521 Enhances MLL-AF9-Dependent Hematopoietic Stem Cell Transformation in Acute Myeloid Leukemias by Altering the Gene Expression Landscape.Int J Mol Sci. 2021 Oct 6;22(19):10814. doi: 10.3390/ijms221910814. Int J Mol Sci. 2021. PMID: 34639154 Free PMC article.
-
SLC38A5 Modulates Ferroptosis to Overcome Gemcitabine Resistance in Pancreatic Cancer.Cells. 2023 Oct 23;12(20):2509. doi: 10.3390/cells12202509. Cells. 2023. PMID: 37887353 Free PMC article.
-
Metabolic Pathways in Breast Cancer Reprograming: An Insight to Non-Coding RNAs.Cells. 2022 Sep 23;11(19):2973. doi: 10.3390/cells11192973. Cells. 2022. PMID: 36230935 Free PMC article. Review.
-
Integration of parallel metabolomics and transcriptomics reveals metabolic patterns in porcine oocytes during maturation.Front Endocrinol (Lausanne). 2023 Feb 1;14:1131256. doi: 10.3389/fendo.2023.1131256. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 36817597 Free PMC article.
-
Role of glutamine and its metabolite ammonia in crosstalk of cancer-associated fibroblasts and cancer cells.Cancer Cell Int. 2021 Sep 9;21(1):479. doi: 10.1186/s12935-021-02121-5. Cancer Cell Int. 2021. PMID: 34503536 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

