Multifaceted Interaction Between Hepatitis B Virus Infection and Lipid Metabolism in Hepatocytes: A Potential Target of Antiviral Therapy for Chronic Hepatitis B
- PMID: 33776969
- PMCID: PMC7991784
- DOI: 10.3389/fmicb.2021.636897
Multifaceted Interaction Between Hepatitis B Virus Infection and Lipid Metabolism in Hepatocytes: A Potential Target of Antiviral Therapy for Chronic Hepatitis B
Abstract
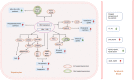
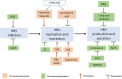
Hepatitis B virus (HBV) is considered a "metabolic virus" and affects many hepatic metabolic pathways. However, how HBV affects lipid metabolism in hepatocytes remains uncertain yet. Accumulating clinical studies suggested that compared to non-HBV-infected controls, chronic HBV infection was associated with lower levels of serum total cholesterol and triglycerides and a lower prevalence of hepatic steatosis. In patients with chronic HBV infection, high ALT level, high body mass index, male gender, or old age was found to be positively correlated with hepatic steatosis. Furthermore, mechanisms of how HBV infection affected hepatic lipid metabolism had also been explored in a number of studies based on cell lines and mouse models. These results demonstrated that HBV replication or expression induced extensive and diverse changes in hepatic lipid metabolism, by not only activating expression of some critical lipogenesis and cholesterolgenesis-related proteins but also upregulating fatty acid oxidation and bile acid synthesis. Moreover, increasing studies found some potential targets to inhibit HBV replication or expression by decreasing or enhancing certain lipid metabolism-related proteins or metabolites. Therefore, in this article, we comprehensively reviewed these publications and revealed the connections between clinical observations and experimental findings to better understand the interaction between hepatic lipid metabolism and HBV infection. However, the available data are far from conclusive, and there is still a long way to go before clarifying the complex interaction between HBV infection and hepatic lipid metabolism.
Keywords: apolipoprotein; chronic hepatitis B; hepatic steatosis; hepatitis B virus; lipid metabolism; metabolic signaling pathway; nuclear factors.
Copyright © 2021 Zhang, Ling, Lei, Peng, Hu and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Impact of hepatitis B virus infection on hepatic metabolic signaling pathway.World J Gastroenterol. 2016 Sep 28;22(36):8161-7. doi: 10.3748/wjg.v22.i36.8161. World J Gastroenterol. 2016. PMID: 27688657 Free PMC article. Review.
-
Hepatitis B Virus X Protein Induces Hepatic Steatosis by Enhancing the Expression of Liver Fatty Acid Binding Protein.J Virol. 2015 Dec 4;90(4):1729-40. doi: 10.1128/JVI.02604-15. Print 2016 Feb 15. J Virol. 2015. PMID: 26637457 Free PMC article.
-
Perilipin2 inhibits the replication of hepatitis B virus deoxyribonucleic acid by regulating autophagy under high-fat conditions.World J Virol. 2023 Dec 25;12(5):296-308. doi: 10.5501/wjv.v12.i5.296. World J Virol. 2023. PMID: 38187502 Free PMC article.
-
Non-alcoholic hepatic steatosis attenuates hepatitis B virus replication in an HBV-immunocompetent mouse model.Hepatol Int. 2018 Sep;12(5):438-446. doi: 10.1007/s12072-018-9877-7. Epub 2018 Jul 5. Hepatol Int. 2018. PMID: 29974410
-
Rethinking the pathogenesis of hepatitis B virus (HBV) infection.J Med Virol. 2015 Dec;87(12):1989-99. doi: 10.1002/jmv.24270. Epub 2015 May 29. J Med Virol. 2015. PMID: 25989114 Review.
Cited by
-
SGIV Induced and Exploited Cellular De Novo Fatty Acid Synthesis for Virus Entry and Replication.Viruses. 2022 Jan 18;14(2):180. doi: 10.3390/v14020180. Viruses. 2022. PMID: 35215774 Free PMC article.
-
Metabolic Regulation of Hepatitis B Virus Infection in HBV-Transgenic Mice.Metabolites. 2022 Mar 25;12(4):287. doi: 10.3390/metabo12040287. Metabolites. 2022. PMID: 35448475 Free PMC article.
-
Interplay between Lipid Metabolism, Lipid Droplets, and DNA Virus Infections.Cells. 2022 Jul 17;11(14):2224. doi: 10.3390/cells11142224. Cells. 2022. PMID: 35883666 Free PMC article. Review.
-
Metabolic Disorders in Patients with Chronic Hepatitis B Virus Infection: Coffee as a Panacea? (ANRS CO22 Hepather Cohort).Antioxidants (Basel). 2022 Feb 14;11(2):379. doi: 10.3390/antiox11020379. Antioxidants (Basel). 2022. PMID: 35204261 Free PMC article.
-
Small hepatitis B virus surface antigen (SHBs) induces dyslipidemia by suppressing apolipoprotein-AII expression through ER stress-mediated modulation of HNF4α and C/EBPγ.J Virol. 2024 Nov 19;98(11):e0123924. doi: 10.1128/jvi.01239-24. Epub 2024 Oct 29. J Virol. 2024. PMID: 39470210
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

