Coronavirus disease 2019 vaccine response in pregnant and lactating women: a cohort study
- PMID: 33775692
- PMCID: PMC7997025
- DOI: 10.1016/j.ajog.2021.03.023
Coronavirus disease 2019 vaccine response in pregnant and lactating women: a cohort study
Abstract
Background: Pregnant and lactating women were excluded from initial coronavirus disease 2019 vaccine trials; thus, data to guide vaccine decision making are lacking.
Objective: This study aimed to evaluate the immunogenicity and reactogenicity of coronavirus disease 2019 messenger RNA vaccination in pregnant and lactating women compared with: (1) nonpregnant controls and (2) natural coronavirus disease 2019 infection in pregnancy.
Study design: A total of 131 reproductive-age vaccine recipients (84 pregnant, 31 lactating, and 16 nonpregnant women) were enrolled in a prospective cohort study at 2 academic medical centers. Titers of severe acute respiratory syndrome coronavirus 2 spike and receptor-binding domain immunoglobulin G, immunoglobulin A, and immunoglobulin M were quantified in participant sera (n=131) and breastmilk (n=31) at baseline, at the second vaccine dose, at 2 to 6 weeks after the second vaccine, and at delivery by Luminex. Umbilical cord sera (n=10) titers were assessed at delivery. Titers were compared with those of pregnant women 4 to 12 weeks from the natural infection (n=37) by enzyme-linked immunosorbent assay. A pseudovirus neutralization assay was used to quantify neutralizing antibody titers for the subset of women who delivered during the study period. Postvaccination symptoms were assessed via questionnaire. Kruskal-Wallis tests and a mixed-effects model, with correction for multiple comparisons, were used to assess differences among groups.
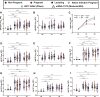
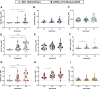
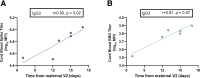
Results: Vaccine-induced antibody titers were equivalent in pregnant and lactating compared with nonpregnant women (pregnant, median, 5.59; interquartile range, 4.68-5.89; lactating, median, 5.74; interquartile range, 5.06-6.22; nonpregnant, median, 5.62; interquartile range, 4.77-5.98, P=.24). All titers were significantly higher than those induced by severe acute respiratory syndrome coronavirus 2 infection during pregnancy (P<.0001). Vaccine-generated antibodies were present in all umbilical cord blood and breastmilk samples. Neutralizing antibody titers were lower in umbilical cord than maternal sera, although this finding did not achieve statistical significance (maternal sera, median, 104.7; interquartile range, 61.2-188.2; cord sera, median, 52.3; interquartile range, 11.7-69.6; P=.05). The second vaccine dose (boost dose) increased severe acute respiratory syndrome coronavirus 2-specific immunoglobulin G, but not immunoglobulin A, in maternal blood and breastmilk. No differences were noted in reactogenicity across the groups.
Conclusion: Coronavirus disease 2019 messenger RNA vaccines generated robust humoral immunity in pregnant and lactating women, with immunogenicity and reactogenicity similar to that observed in nonpregnant women. Vaccine-induced immune responses were statistically significantly greater than the response to natural infection. Immune transfer to neonates occurred via placenta and breastmilk.
Keywords: COVID-19 vaccine; antibodies; breastfeeding; breastmilk; cord blood; mRNA; maternal immunity; neonatal immunity; pregnancy.
Copyright © 2021 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures




Update of
-
COVID-19 vaccine response in pregnant and lactating women: a cohort study.medRxiv [Preprint]. 2021 Mar 8:2021.03.07.21253094. doi: 10.1101/2021.03.07.21253094. medRxiv. 2021. Update in: Am J Obstet Gynecol. 2021 Sep;225(3):303.e1-303.e17. doi: 10.1016/j.ajog.2021.03.023 PMID: 33758889 Free PMC article. Updated. Preprint.
Comment in
-
Accommodating vaccine preferences among women of childbearing age.Am J Obstet Gynecol. 2021 Dec;225(6):697-699. doi: 10.1016/j.ajog.2021.07.017. Epub 2021 Jul 31. Am J Obstet Gynecol. 2021. PMID: 34343503 Free PMC article. No abstract available.
Similar articles
-
COVID-19 vaccine response in pregnant and lactating women: a cohort study.medRxiv [Preprint]. 2021 Mar 8:2021.03.07.21253094. doi: 10.1101/2021.03.07.21253094. medRxiv. 2021. Update in: Am J Obstet Gynecol. 2021 Sep;225(3):303.e1-303.e17. doi: 10.1016/j.ajog.2021.03.023 PMID: 33758889 Free PMC article. Updated. Preprint.
-
Longitudinal antibody response kinetics following SARS-CoV-2 messenger RNA vaccination in pregnant and nonpregnant persons.Am J Obstet Gynecol MFM. 2023 Feb;5(2):100796. doi: 10.1016/j.ajogmf.2022.100796. Epub 2022 Nov 2. Am J Obstet Gynecol MFM. 2023. PMID: 36334723 Free PMC article.
-
Pregnancy alters interleukin-1 beta expression and antiviral antibody responses during severe acute respiratory syndrome coronavirus 2 infection.Am J Obstet Gynecol. 2021 Sep;225(3):301.e1-301.e14. doi: 10.1016/j.ajog.2021.03.028. Epub 2021 Mar 30. Am J Obstet Gynecol. 2021. PMID: 33798476 Free PMC article.
-
Is the Immunization of Pregnant Women against COVID-19 Justified?Vaccines (Basel). 2021 Aug 30;9(9):970. doi: 10.3390/vaccines9090970. Vaccines (Basel). 2021. PMID: 34579207 Free PMC article. Review.
-
Advancing Clinical Research with Pregnant and Lactating Populations: Overcoming Real and Perceived Liability Risks.Washington (DC): National Academies Press (US); 2024 May 24. Washington (DC): National Academies Press (US); 2024 May 24. PMID: 38648302 Free Books & Documents. Review.
Cited by
-
Factors Associated with Anti-SARS-CoV-2 Vaccine Acceptance among Pregnant Women: Data from Outpatient Women Experiencing High-Risk Pregnancy.Vaccines (Basel). 2023 Feb 16;11(2):454. doi: 10.3390/vaccines11020454. Vaccines (Basel). 2023. PMID: 36851330 Free PMC article.
-
Systematic review and meta-analysis of the effectiveness and perinatal outcomes of COVID-19 vaccination in pregnancy.Nat Commun. 2022 May 10;13(1):2414. doi: 10.1038/s41467-022-30052-w. Nat Commun. 2022. PMID: 35538060 Free PMC article.
-
Preserved recognition of Omicron spike following COVID-19 messenger RNA vaccination in pregnancy.Am J Obstet Gynecol. 2022 Sep;227(3):493.e1-493.e7. doi: 10.1016/j.ajog.2022.04.009. Epub 2022 Apr 14. Am J Obstet Gynecol. 2022. PMID: 35430229 Free PMC article.
-
COVID-19 vaccines: Considering sex differences in efficacy and safety.Contemp Clin Trials. 2022 Apr;115:106700. doi: 10.1016/j.cct.2022.106700. Epub 2022 Feb 8. Contemp Clin Trials. 2022. PMID: 35149232 Free PMC article. Review.
-
COVID-19 vaccination in pregnancy.Am J Obstet Gynecol. 2022 Aug;227(2):136-147. doi: 10.1016/j.ajog.2022.05.020. Epub 2022 May 11. Am J Obstet Gynecol. 2022. PMID: 35568189 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

