The antigenic anatomy of SARS-CoV-2 receptor binding domain
- PMID: 33756110
- PMCID: PMC7891125
- DOI: 10.1016/j.cell.2021.02.032
The antigenic anatomy of SARS-CoV-2 receptor binding domain
Abstract
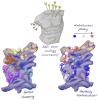
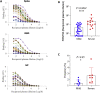
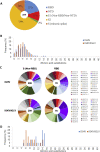
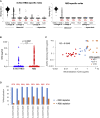
Antibodies are crucial to immune protection against SARS-CoV-2, with some in emergency use as therapeutics. Here, we identify 377 human monoclonal antibodies (mAbs) recognizing the virus spike and focus mainly on 80 that bind the receptor binding domain (RBD). We devise a competition data-driven method to map RBD binding sites. We find that although antibody binding sites are widely dispersed, neutralizing antibody binding is focused, with nearly all highly inhibitory mAbs (IC50 < 0.1 μg/mL) blocking receptor interaction, except for one that binds a unique epitope in the N-terminal domain. Many of these neutralizing mAbs use public V-genes and are close to germline. We dissect the structural basis of recognition for this large panel of antibodies through X-ray crystallography and cryoelectron microscopy of 19 Fab-antigen structures. We find novel binding modes for some potently inhibitory antibodies and demonstrate that strongly neutralizing mAbs protect, prophylactically or therapeutically, in animal models.
Keywords: coronavirus, SARS-CoV-2, anti-RBD antibody, receptor binding domain, antibody, immune responses, virus structure.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests M.S.D. is a consultant for Inbios, Vir Biotechnology, NGM Biopharmaceuticals, and Carnival Corporation and is on the Scientific Advisory Boards of Moderna and Immunome. The M.S.D. laboratory has received unrelated funding support in sponsored research agreements from Moderna, Vir Biotechnology, and Emergent BioSolutions. G.R.S. sits on the GSK Vaccines Scientific Advisory Board. A.J.P. is Chair of UK Department Health and Social Care’s (DHSC) Joint Committee on Vaccination & Immunisation (JCVI), but does not chair or participate in the JCVI COVID19 committee, and is a member of the WHO’s SAGE. The views expressed in this article do not necessarily represent the views of DHSC, JCVI, NIHR, or WHO. The University of Oxford has entered into a partnership with AstraZeneca on coronavirus vaccine development. All other authors declare no competing financial interests. The University of Oxford has protected intellectual property disclosed in this publication.
Figures










Similar articles
-
Structural Basis of a Human Neutralizing Antibody Specific to the SARS-CoV-2 Spike Protein Receptor-Binding Domain.Microbiol Spectr. 2021 Oct 31;9(2):e0135221. doi: 10.1128/Spectrum.01352-21. Epub 2021 Oct 13. Microbiol Spectr. 2021. PMID: 34643438 Free PMC article.
-
Isolation and Characterization of Mouse Monoclonal Antibodies That Neutralize SARS-CoV-2 and Its Variants of Concern Alpha, Beta, Gamma and Delta by Binding Conformational Epitopes of Glycosylated RBD With High Potency.Front Immunol. 2021 Oct 26;12:750386. doi: 10.3389/fimmu.2021.750386. eCollection 2021. Front Immunol. 2021. PMID: 34764961 Free PMC article.
-
The antibody response to SARS-CoV-2 Beta underscores the antigenic distance to other variants.Cell Host Microbe. 2022 Jan 12;30(1):53-68.e12. doi: 10.1016/j.chom.2021.11.013. Epub 2021 Nov 27. Cell Host Microbe. 2022. PMID: 34921776 Free PMC article.
-
Targeting SARS-CoV2 Spike Protein Receptor Binding Domain by Therapeutic Antibodies.Biomed Pharmacother. 2020 Oct;130:110559. doi: 10.1016/j.biopha.2020.110559. Epub 2020 Aug 1. Biomed Pharmacother. 2020. PMID: 32768882 Free PMC article. Review.
-
Structural Analysis of Neutralizing Epitopes of the SARS-CoV-2 Spike to Guide Therapy and Vaccine Design Strategies.Viruses. 2021 Jan 19;13(1):134. doi: 10.3390/v13010134. Viruses. 2021. PMID: 33477902 Free PMC article. Review.
Cited by
-
Resistance of SARS-CoV-2 variants to neutralization by monoclonal and serum-derived polyclonal antibodies.Nat Med. 2021 Apr;27(4):717-726. doi: 10.1038/s41591-021-01294-w. Epub 2021 Mar 4. Nat Med. 2021. PMID: 33664494 Free PMC article.
-
Antibodies elicited by mRNA-1273 vaccination bind more broadly to the receptor binding domain than do those from SARS-CoV-2 infection.Sci Transl Med. 2021 Jun 30;13(600):eabi9915. doi: 10.1126/scitranslmed.abi9915. Epub 2021 Jun 8. Sci Transl Med. 2021. PMID: 34103407 Free PMC article.
-
Prerequisite for COVID-19 Prediction: A Review on Factors Affecting the Infection Rate.Int J Environ Res Public Health. 2022 Oct 11;19(20):12997. doi: 10.3390/ijerph192012997. Int J Environ Res Public Health. 2022. PMID: 36293576 Free PMC article. Review.
-
A cryptic site in class 5 epitope of SARS-CoV-2 RBD maintains highly conservation across natural isolates.iScience. 2024 Jun 11;27(7):110208. doi: 10.1016/j.isci.2024.110208. eCollection 2024 Jul 19. iScience. 2024. PMID: 39015149 Free PMC article.
-
Isolation, characterization, and structure-based engineering of a neutralizing nanobody against SARS-CoV-2.Int J Biol Macromol. 2022 Jun 1;209(Pt A):1379-1388. doi: 10.1016/j.ijbiomac.2022.04.096. Epub 2022 Apr 20. Int J Biol Macromol. 2022. PMID: 35460753 Free PMC article.
References
-
- Aricescu A.R., Lu W., Jones E.Y. A time- and cost-efficient system for high-level protein production in mammalian cells. Acta Crystallogr Sect D Biol Crystallogr. 2006;62:1243–1250. - PubMed
-
- Barnes C.O., West A.P., Jr., Huey-Tubman K.E., Hoffmann M.A.G., Sharaf N.G., Hoffman P.R., Koranda N., Gristick H.B., Gaebler C., Muecksch F. Structures of Human Antibodies Bound to SARS-CoV-2 Spike Reveal Common Epitopes and Recurrent Features of Antibodies. Cell. 2020;182:828–842.e16. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 203224/Z/16/Z/WT_/Wellcome Trust/United Kingdom
- R01 AI157155/AI/NIAID NIH HHS/United States
- MR/N00065X/1/MRC_/Medical Research Council/United Kingdom
- MR/V001329/1/MRC_/Medical Research Council/United Kingdom
- T32 AI007172/AI/NIAID NIH HHS/United States
- MC_PC_19060/MRC_/Medical Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- MC_PC_20016/MRC_/Medical Research Council/United Kingdom
- 109965/Z/15/Z/WT_/Wellcome Trust/United Kingdom
- MC_UU_00008/11/MRC_/Medical Research Council/United Kingdom
- MC_PC_17137/MRC_/Medical Research Council/United Kingdom
- MR/L018942/1/MRC_/Medical Research Council/United Kingdom
- G0600520/MRC_/Medical Research Council/United Kingdom
- G1001046/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

