Interrogating the Small Intestine Tuft Cell-ILC2 Circuit Using In Vivo Manipulations
- PMID: 33740294
- PMCID: PMC8082719
- DOI: 10.1002/cpz1.77
Interrogating the Small Intestine Tuft Cell-ILC2 Circuit Using In Vivo Manipulations
Erratum in
-
Corrections.Curr Protoc. 2021 Jul;1(7):e205. doi: 10.1002/cpz1.205. Curr Protoc. 2021. PMID: 34242484 No abstract available.
-
Group Correction Statement (Conflict of Interest Statements).Curr Protoc. 2022 Aug;2(8):e551. doi: 10.1002/cpz1.551. Curr Protoc. 2022. PMID: 36005903 Free PMC article. No abstract available.
Abstract
Recent findings position tuft cells as key mediators of intestinal immunity through their production of the cytokine interleukin (IL)-25 and activation of group 2 innate lymphoid cells (ILC2s). Though tuft cells are found in numerous epithelial tissues, their phenotype and function have been best characterized in the small intestine, where robust in vivo techniques have enabled the dissection of their cellular function, ontogeny, and key signaling pathways. We describe methods for the identification, quantification, and manipulation of tuft cells, focusing on analysis of ILC2s as a readout of tuft cell function. © 2021 Wiley Periodicals LLC. Basic Protocol 1: Ex vivo analysis of small intestinal tuft cells and ILC2 by flow cytometry Alternate Protocol: Ex vivo analysis of small intestinal tuft cells and ILC2 by flow cytometry in the context of type 2 inflammation Basic Protocol 2: Ex vivo analysis of small intestinal tuft cells by imaging of intestinal Swiss roll Basic Protocol 3: Tuft-ILC2 circuit activation by oral gavage of adult Nippostrongylus brasiliensis worms Basic Protocol 4: Circuit activation by colonization with Tritrichomonas spp. Basic Protocol 5: Circuit activation by treatment with succinate in drinking water Basic Protocol 6: Circuit activation by treatment with recombinant IL-25.
Keywords: IL-25; Nippostrongylus brasiliensis; Tritrichomonas spp; group 2 innate lymphoid cells (ILC2s); helminth infection; parasites; small intestine; succinate; tuft cells; type 2 cytokine signaling; type 2 immunity.
© 2021 Wiley Periodicals LLC.
Figures

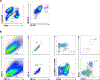




Similar articles
-
Detection of Succinate by Intestinal Tuft Cells Triggers a Type 2 Innate Immune Circuit.Immunity. 2018 Jul 17;49(1):33-41.e7. doi: 10.1016/j.immuni.2018.06.016. Immunity. 2018. PMID: 30021144 Free PMC article.
-
Tuft-Cell-Derived Leukotrienes Drive Rapid Anti-helminth Immunity in the Small Intestine but Are Dispensable for Anti-protist Immunity.Immunity. 2020 Mar 17;52(3):528-541.e7. doi: 10.1016/j.immuni.2020.02.005. Epub 2020 Mar 10. Immunity. 2020. PMID: 32160525 Free PMC article.
-
A Metabolite-Triggered Tuft Cell-ILC2 Circuit Drives Small Intestinal Remodeling.Cell. 2018 Jul 12;174(2):271-284.e14. doi: 10.1016/j.cell.2018.05.014. Epub 2018 Jun 7. Cell. 2018. PMID: 29887373 Free PMC article.
-
Possible connection between intestinal tuft cells, ILC2s and obesity.Front Immunol. 2024 Jan 12;14:1266667. doi: 10.3389/fimmu.2023.1266667. eCollection 2023. Front Immunol. 2024. PMID: 38283340 Free PMC article. Review.
-
Heterogeneity of ILC2s in the Intestine; Homeostasis and Pathology.Front Immunol. 2022 May 30;13:867351. doi: 10.3389/fimmu.2022.867351. eCollection 2022. Front Immunol. 2022. PMID: 35707544 Free PMC article. Review.
Cited by
-
New insights into tuft cell formation: Implications for structure-function relationships.Curr Opin Cell Biol. 2022 Jun;76:102082. doi: 10.1016/j.ceb.2022.102082. Epub 2022 Apr 22. Curr Opin Cell Biol. 2022. PMID: 35468541 Free PMC article. Review.
-
Bile acid-sensitive tuft cells regulate biliary neutrophil influx.Sci Immunol. 2022 Mar 4;7(69):eabj1080. doi: 10.1126/sciimmunol.abj1080. Epub 2022 Mar 4. Sci Immunol. 2022. PMID: 35245089 Free PMC article.
-
Tuft cells in the intestine, immunity and beyond.Nat Rev Gastroenterol Hepatol. 2024 Dec;21(12):852-868. doi: 10.1038/s41575-024-00978-1. Epub 2024 Sep 26. Nat Rev Gastroenterol Hepatol. 2024. PMID: 39327439 Review.
-
The Role of Intestinal Microbial Metabolites in the Immunity of Equine Animals Infected With Horse Botflies.Front Vet Sci. 2022 Jun 22;9:832062. doi: 10.3389/fvets.2022.832062. eCollection 2022. Front Vet Sci. 2022. PMID: 35812868 Free PMC article.
-
Tuft Cells and Their Role in Intestinal Diseases.Front Immunol. 2022 Feb 14;13:822867. doi: 10.3389/fimmu.2022.822867. eCollection 2022. Front Immunol. 2022. PMID: 35237268 Free PMC article. Review.
References
-
- Bezençon C, Fürholz A, Raymond F, Mansourian R, Métairon S, Le Coutre J, & Damak S. (2008). Murine intestinal cells expressing Trpm5 are mostly brush cells and express markers of neuronal and inflammatory cells. The Journal of Comparative Neurology, 509(5), 514–525. doi:10.1002/cne.21768 - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

