High-Dose Intravenous Immunoglobulin in Severe Coronavirus Disease 2019: A Multicenter Retrospective Study in China
- PMID: 33679771
- PMCID: PMC7933558
- DOI: 10.3389/fimmu.2021.627844
High-Dose Intravenous Immunoglobulin in Severe Coronavirus Disease 2019: A Multicenter Retrospective Study in China
Erratum in
-
Corrigendum: High-Dose Intravenous Immunoglobulin in Severe Coronavirus Disease 2019: A Multicenter Retrospective Study in China.Front Immunol. 2021 Mar 22;12:671443. doi: 10.3389/fimmu.2021.671443. eCollection 2021. Front Immunol. 2021. PMID: 33831136 Free PMC article.
Abstract
Background: The effective treatment of coronavirus disease 2019 (COVID-19) remains unclear. We reported successful use of high-dose intravenous immunoglobulin (IVIg) in cases of severe COVID-19, but evidence from larger case series is still lacking.
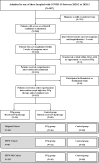
Methods: A multi-center retrospective study was conducted to evaluate the effectiveness of IVIg administered within two weeks of disease onset at a total dose of 2 g/kg body weight, in addition to standard care. The primary endpoint was 28-day mortality. Efficacy of high-dose IVIg was assessed by using the Cox proportional hazards regression model and the Kaplan-Meier curve adjusted by inverse probability of treatment weighting (IPTW) analysis, and IPTW after multiple imputation (MI) analysis.
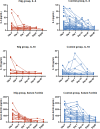
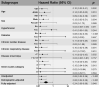
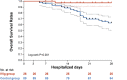
Results: Overall, 26 patients who received high-dose IVIg with standard therapy and 89 patients who received standard therapy only were enrolled in this study. The IVIg group was associated with a lower 28-day mortality rate and less time to normalization of inflammatory markers including IL-6, IL-10, and ferritin compared with the control. The adjusted HR of 28-day mortality in high-dose IVIg group was 0.24 (95% CI 0.06-0.99, p<0.001) in IPTW model, and 0.27 (95% CI 0.10-0.57, p=0.031) in IPTW-MI model. In subgroup analysis, patients with no comorbidities or treated in the first week of disease were associated with more benefit from high-dose IVIg.
Conclusions: High-dose IVIg administered in severe COVID-19 patients within 14 days of onset was linked to reduced 28-day mortality, more prominent with those having no comorbidities or treated at earlier stage.
Keywords: 28-day mortality; COVID-19; high-dose intravenous immunoglobulin; immunomodulation; inflammatory markers.
Copyright © 2021 Cao, Liu, Hong, Ma, Zhang, Lin, Han, Xiong, Liu, Ruan and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Intravenous Immunoglobulin Therapy for Critically Ill COVID-19 Patients With Different Inflammatory Phenotypes: A Multicenter, Retrospective Study.Front Immunol. 2022 Jan 27;12:738532. doi: 10.3389/fimmu.2021.738532. eCollection 2021. Front Immunol. 2022. PMID: 35154067 Free PMC article.
-
Efficacy evaluation of intravenous immunoglobulin in non-severe patients with COVID-19: A retrospective cohort study based on propensity score matching.Int J Infect Dis. 2021 Apr;105:525-531. doi: 10.1016/j.ijid.2021.01.009. Epub 2021 Jan 9. Int J Infect Dis. 2021. PMID: 33434674 Free PMC article.
-
A single center experience of intravenous immunoglobulin treatment in Covid-19.Int Immunopharmacol. 2021 Sep;98:107891. doi: 10.1016/j.intimp.2021.107891. Epub 2021 Jun 14. Int Immunopharmacol. 2021. PMID: 34153671 Free PMC article.
-
Intravenous immunoglobulin as an important adjunct in the prevention and therapy of coronavirus 2019 disease.Scand J Immunol. 2021 Nov;94(5):e13101. doi: 10.1111/sji.13101. Epub 2021 Sep 16. Scand J Immunol. 2021. PMID: 34940980 Free PMC article. Review.
-
Intravenous immunoglobulins (IVIG) in severe/critical COVID-19 adult patients.Biomed Pharmacother. 2023 Jul;163:114851. doi: 10.1016/j.biopha.2023.114851. Epub 2023 May 5. Biomed Pharmacother. 2023. PMID: 37167723 Free PMC article. Review.
Cited by
-
Intravenous Immunoglobulin Therapy for Critically Ill COVID-19 Patients With Different Inflammatory Phenotypes: A Multicenter, Retrospective Study.Front Immunol. 2022 Jan 27;12:738532. doi: 10.3389/fimmu.2021.738532. eCollection 2021. Front Immunol. 2022. PMID: 35154067 Free PMC article.
-
Current development of severe acute respiratory syndrome coronavirus 2 neutralizing antibodies (Review).Mol Med Rep. 2024 Aug;30(2):148. doi: 10.3892/mmr.2024.13272. Epub 2024 Jun 28. Mol Med Rep. 2024. PMID: 38940338 Free PMC article. Review.
-
The Functional Role of IgA in the IgM/IgA-Enriched Immunoglobulin Preparation Trimodulin.Biomedicines. 2021 Dec 3;9(12):1828. doi: 10.3390/biomedicines9121828. Biomedicines. 2021. PMID: 34944644 Free PMC article.
-
From Cytokine Storm to Cytokine Breeze: Did Lessons Learned from Immunopathogenesis Improve Immunomodulatory Treatment of Moderate-to-Severe COVID-19?Biomedicines. 2022 Oct 18;10(10):2620. doi: 10.3390/biomedicines10102620. Biomedicines. 2022. PMID: 36289881 Free PMC article. Review.
-
COVID-19-specific transcriptomic signature detectable in blood across multiple cohorts.Front Genet. 2022 Aug 5;13:929887. doi: 10.3389/fgene.2022.929887. eCollection 2022. Front Genet. 2022. PMID: 35991542 Free PMC article.
References
-
- World Health Organization . Coronavirus disease (COVID-2019) situation reports (2020). Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situatio.... [Accessed Oct 22, 2020].
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

