An Oncolytic Adenovirus Encoding SA-4-1BBL Adjuvant Fused to HPV-16 E7 Antigen Produces a Specific Antitumor Effect in a Cancer Mouse Model
- PMID: 33673295
- PMCID: PMC7917608
- DOI: 10.3390/vaccines9020149
An Oncolytic Adenovirus Encoding SA-4-1BBL Adjuvant Fused to HPV-16 E7 Antigen Produces a Specific Antitumor Effect in a Cancer Mouse Model
Abstract
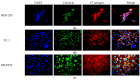
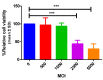
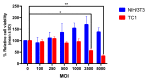
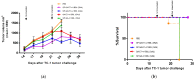
Human papillomaviruses (HPVs) are responsible for about 25% of cancer cases worldwide. HPV-16 E7 antigen is a tumor-associated antigen (TAA) commonly expressed in HPV-induced tumors; however, it has low immunogenicity. The interaction of 4-1BBL with its receptor induces pleiotropic effects on innate, adaptive, and regulatory immunity and, if fused to TAAs in DNA vaccines, can improve the antitumor response; however, there is low transfection and antitumor efficiency. Oncolytic virotherapy is promising for antitumor gene therapy as it can be selectively replicated in tumor cells, inducing cell lysis, and furthermore, tumor cell debris can be taken in by immune cells to potentiate antitumor responses. In this study, we expressed the immunomodulatory molecule SA-4-1BBL fused to E7 on an oncolytic adenovirus (OAd) system. In vitro infection of TC-1 tumor cells and NIH-3T3 non-tumor cells with SA/E7/4-1BBL OAd demonstrated that only tumor cells are selectively destroyed. Moreover, protein expression is targeted to the endoplasmic reticulum in both cell lines when a signal peptide (SP) is added. Finally, in an HPV-induced cancer murine model, the therapeutic oncolytic activity of OAd can be detected, and this can be improved when fused to E7 and SP.
Keywords: 4-1BBL; HPV-16 antigen; cancer vaccines; oncolytic virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Long-term antigen-specific immune response by an oncolytic adenovirus encoding SP-SA-E7-4-1BBL in HPV-16 cancer model.Mol Biol Rep. 2024 Mar 9;51(1):408. doi: 10.1007/s11033-024-09303-0. Mol Biol Rep. 2024. PMID: 38460043
-
A DNA Vaccine Encoding SA-4-1BBL Fused to HPV-16 E7 Antigen Has Prophylactic and Therapeutic Efficacy in a Cervical Cancer Mouse Model.Cancers (Basel). 2019 Jan 15;11(1):96. doi: 10.3390/cancers11010096. Cancers (Basel). 2019. PMID: 30650588 Free PMC article.
-
SA-4-1BBL as the immunomodulatory component of a HPV-16 E7 protein based vaccine shows robust therapeutic efficacy in a mouse cervical cancer model.Vaccine. 2010 Aug 16;28(36):5794-802. doi: 10.1016/j.vaccine.2010.06.073. Epub 2010 Jul 4. Vaccine. 2010. PMID: 20603135 Free PMC article.
-
Endoplasmic reticulum stress enhances the antigen-specific T cell immune responses and therapeutic antitumor effects generated by therapeutic HPV vaccines.J Biomed Sci. 2019 May 27;26(1):41. doi: 10.1186/s12929-019-0536-7. J Biomed Sci. 2019. PMID: 31133013 Free PMC article.
-
CD4+ T cells play a critical role in the generation of primary and memory antitumor immune responses elicited by SA-4-1BBL and TAA-based vaccines in mouse tumor models.PLoS One. 2013 Sep 16;8(9):e73145. doi: 10.1371/journal.pone.0073145. eCollection 2013. PLoS One. 2013. PMID: 24066030 Free PMC article.
Cited by
-
Harnessing adenovirus in cancer immunotherapy: evoking cellular immunity and targeting delivery in cell-specific manner.Biomark Res. 2024 Mar 25;12(1):36. doi: 10.1186/s40364-024-00581-1. Biomark Res. 2024. PMID: 38528632 Free PMC article. Review.
-
Long-term antigen-specific immune response by an oncolytic adenovirus encoding SP-SA-E7-4-1BBL in HPV-16 cancer model.Mol Biol Rep. 2024 Mar 9;51(1):408. doi: 10.1007/s11033-024-09303-0. Mol Biol Rep. 2024. PMID: 38460043
-
Using PAMPs and DAMPs as adjuvants in cancer vaccines.Hum Vaccin Immunother. 2021 Dec 2;17(12):5546-5557. doi: 10.1080/21645515.2021.1964316. Epub 2021 Sep 14. Hum Vaccin Immunother. 2021. PMID: 34520322 Free PMC article. Review.
-
Engineering strategies to enhance oncolytic viruses in cancer immunotherapy.Signal Transduct Target Ther. 2022 Apr 6;7(1):117. doi: 10.1038/s41392-022-00951-x. Signal Transduct Target Ther. 2022. PMID: 35387984 Free PMC article. Review.
-
Development of Next-Generation Vaccines.Vaccines (Basel). 2022 Feb 10;10(2):274. doi: 10.3390/vaccines10020274. Vaccines (Basel). 2022. PMID: 35214730 Free PMC article.
References
-
- Cáncer. [(accessed on 6 January 2020)]; Available online: https://www.who.int/es/news-room/fact-sheets/detail/cancer.
-
- Lin K.-Y., Guarnieri F.G., Staveley-O’Carroll K.F., Levitsky H.I., August J.T., Pardoll D.M., Wu T.-C. Treatment of Established Tumors with a Novel Vaccine That Enhances Major Histocompatibility Class II Presentation of Tumor Antigen. Cancer Res. 1996;56:21–26. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

