Notch signaling drives development of Barrett's metaplasia from Dclk1-positive epithelial tuft cells in the murine gastric mucosa
- PMID: 33627749
- PMCID: PMC7904766
- DOI: 10.1038/s41598-021-84011-4
Notch signaling drives development of Barrett's metaplasia from Dclk1-positive epithelial tuft cells in the murine gastric mucosa
Abstract
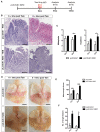
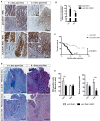
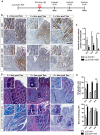
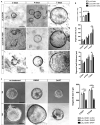
Barrett's esophagus (BE) is a precursor to esophageal adenocarcinoma (EAC), but its cellular origin and mechanism of neoplastic progression remain unresolved. Notch signaling, which plays a key role in regulating intestinal stem cell maintenance, has been implicated in a number of cancers. The kinase Dclk1 labels epithelial post-mitotic tuft cells at the squamo-columnar junction (SCJ), and has also been proposed to contribute to epithelial tumor growth. Here, we find that genetic activation of intracellular Notch signaling in epithelial Dclk1-positive tuft cells resulted in the accelerated development of metaplasia and dysplasia in a mouse model of BE (pL2.Dclk1.N2IC mice). In contrast, genetic ablation of Notch receptor 2 in Dclk1-positive cells delayed BE progression (pL2.Dclk1.N2fl mice), and led to increased secretory cell differentiation. The accelerated BE progression in pL2.Dclk1.N2IC mice correlated with changes to the transcriptomic landscape, most notably for the activation of oncogenic, proliferative pathways in BE tissues, in contrast to upregulated Wnt signalling in pL2.Dclk1.N2fl mice. Collectively, our data show that Notch activation in Dclk1-positive tuft cells in the gastric cardia can contribute to BE development.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Notch Signaling Mediates Differentiation in Barrett's Esophagus and Promotes Progression to Adenocarcinoma.Gastroenterology. 2020 Aug;159(2):575-590. doi: 10.1053/j.gastro.2020.04.033. Epub 2020 Apr 20. Gastroenterology. 2020. PMID: 32325086 Free PMC article.
-
Inhibition of Notch signaling enhances transdifferentiation of the esophageal squamous epithelium towards a Barrett's-like metaplasia via KLF4.Cell Cycle. 2014;13(24):3857-66. doi: 10.4161/15384101.2014.972875. Cell Cycle. 2014. PMID: 25558829 Free PMC article.
-
Aberrant epithelial-mesenchymal Hedgehog signaling characterizes Barrett's metaplasia.Gastroenterology. 2010 May;138(5):1810-22. doi: 10.1053/j.gastro.2010.01.048. Epub 2010 Feb 4. Gastroenterology. 2010. PMID: 20138038 Free PMC article.
-
Origins of Metaplasia in Barrett's Esophagus: Is this an Esophageal Stem or Progenitor Cell Disease?Dig Dis Sci. 2018 Aug;63(8):2005-2012. doi: 10.1007/s10620-018-5069-5. Dig Dis Sci. 2018. PMID: 29675663 Free PMC article. Review.
-
The diagnosis and management of Barrett's esophagus.Adv Surg. 1999;33:29-68. Adv Surg. 1999. PMID: 10572561 Review.
Cited by
-
Cranberry Polyphenols in Esophageal Cancer Inhibition: New Insights.Nutrients. 2022 Feb 24;14(5):969. doi: 10.3390/nu14050969. Nutrients. 2022. PMID: 35267943 Free PMC article.
-
IL-13-programmed airway tuft cells produce PGE2, which promotes CFTR-dependent mucociliary function.JCI Insight. 2022 Jul 8;7(13):e159832. doi: 10.1172/jci.insight.159832. JCI Insight. 2022. PMID: 35608904 Free PMC article.
-
Tuft Cells and Their Role in Intestinal Diseases.Front Immunol. 2022 Feb 14;13:822867. doi: 10.3389/fimmu.2022.822867. eCollection 2022. Front Immunol. 2022. PMID: 35237268 Free PMC article. Review.
-
Tuft Cells: Context- and Tissue-Specific Programming for a Conserved Cell Lineage.Annu Rev Pathol. 2023 Jan 24;18:311-335. doi: 10.1146/annurev-pathol-042320-112212. Epub 2022 Nov 9. Annu Rev Pathol. 2023. PMID: 36351364 Free PMC article. Review.
-
Pleiotropic effects of DCLK1 in cancer and cancer stem cells.Front Mol Biosci. 2022 Sep 26;9:965730. doi: 10.3389/fmolb.2022.965730. eCollection 2022. Front Mol Biosci. 2022. PMID: 36250024 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

